Professional Documents
Culture Documents
Churg Strauss Syndrome
Uploaded by
Muchamad ZubaidOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Churg Strauss Syndrome
Uploaded by
Muchamad ZubaidCopyright:
Available Formats
Anatomic Pathology / CHURG-STRAUSS SYNDROME
Diagnostic Features and Differential Diagnosis of Churg-Strauss Syndrome in the Lung A Review
Anna-Luise A. Katzenstein, MD
Key Words: Churg-Strauss syndrome; Allergic angiitis and granulomatosis; Eosinophilic pneumonia; Wegener granulomatosis; Eosinophilic lung disease
Abstract
Churg-Strauss syndrome is a rare systemic vasculitis occurring in patients with asthma and blood eosinophilia. Lungs, skin, and nervous system are the most common sites of involvement, although many other organs are affected frequently. The diagnosis often is established from clinical findings or biopsy of extrapulmonary sites, and lung biopsy is performed infrequently. The classic pathologic findings in the lung include a combination of eosinophilic pneumonia, granulomatous inflammation, and vasculitis. All 3 features may not be present in every case, however, and diagnosis often requires careful correlation of the clinical and pathologic findings. The differential diagnosis in the lung includes diseases that are associated with eosinophil infiltrates or a combination of eosinophil infiltrates and granulomatous inflammation. Distinguishing these various diseases from Churg-Strauss syndrome is especially important, since many are more common than Churg-Strauss syndrome, and treatment is usually different.
Churg-Strauss syndrome (also known as allergic angiitis and granulomatosis) is a rare systemic vasculitis that occurs exclusively in people with asthma and is associated with blood and tissue eosinophilia. A recent consensus conference on the nomenclature of the systemic vasculitides defined it as eosinophil-rich and granulomatous inflammation involving the respiratory tract, and necrotizing vasculitis affecting small to medium-sized vessels, and associated with asthma and eosinophilia.1 Clinical criteria for diagnosis include the combination of asthma, blood eosinophilia greater than 1,500/L (1.5 109/L), and evidence of vasculitis involving 2 or more extrapulmonary organs.2 Biopsies may be performed to confirm the diagnosis and most commonly are from skin, nerve, or muscle. Lung biopsies are performed infrequently.
Clinical Features
Churg-Strauss syndrome occurs mainly in middle-aged adults. The mean age of onset is 38 years, although there is a wide age range, and the disease can affect children and elderly people.3 Overall, there seems to be no sex preference, although a few series have noted a male predominance.4,5 Patients have a history of asthma that is most often of adult onset, usually in the fourth decade. Allergic rhinitis, nasal polyps, and sinusitis are common accompanying features. Some investigators have suggested that there are 3 phases to the disease: a prodromal phase with allergic rhinitis that may be present for years and is followed by asthma, a second phase characterized by blood and tissue eosinophilia with eosinophilic pneumonia or eosinophilic gastroenteritis, and a third phase characterized by systemic vasculitis.2 The disease has been reported in people with asthma treated with
Am J Clin Pathol 2000;114:767-772
767
American Society of Clinical Pathologists
Katzenstein / CHURG-STRAUSS SYNDROME
leukotriene receptor antagonists, such as montelukast and zafirlukast.6 It is thought that the decreased corticosteroid dosage needed to control asthma symptoms in patients receiving leukotriene receptor antagonists unmasks an underlying vasculitis that previously had been controlled by the corticosteroids. Systemic symptoms, including fever and weight loss, are observed in most patients at the initial examination. Pulmonary infiltrates, cutaneous lesions, and neuropathy are common manifestations, each occurring in two thirds or more of patients.3 The most common chest radiographic findings include transient patchy alveolar opacities, while diffuse interstitial infiltrates or nodular densities occur infrequently.7 Purpura and subcutaneous nodules are the usual skin manifestations, while peripheral neuropathy, especially mononeuritis multiplex, is the characteristic neurologic finding. Other common sites of involvement include the gastrointestinal system with abdominal pain, diarrhea, or bleeding; heart with congestive heart failure, cardiomyopathy, and pericardial effusion; and kidney with focal segmental glomerulonephritis that is generally mild. Blood eosinophilia is present in all patients and is usually extremely high, averaging greater than 1,000/L (1 109/L). Approximately two thirds of patients have a positive P-ANCA (antineutrophil cytoplasmic autoantibody) test result.3,8 Most patients are treated with corticosteroids, although immunosuppressive drugs, usually cyclophosphamide, may be added in some cases.3 The prognosis is good, with remission occurring in the majority of patients. Cardiac involvement with myocardial infarction or congestive heart failure is the most common cause of death.
Table 1 Pathologic Diagnosis of Churg-Strauss Syndrome
Classic diagnostic findings: Eosinophilic pneumonia, necrotizing vasculitis, and granulomatous inflammation Highly suggestive findings (need clinicopathologic correlation): Eosinophilic pneumonia and necrotizing vasculitis Suggestive findings (need clinicopathologic correlation): Eosinophilic pneumonia and parenchymal necrosis Common, but not diagnostic finding (more likely an isolated finding unrelated to Churg-Strauss syndrome): Eosinophilic pneumonia
Pathologic Findings
There are surprisingly few detailed pathologic descriptions of the lung findings in Churg-Strauss syndrome.9-12 Part of the problem is that tissue biopsy is not necessary for diagnosis in every case, and sites other than lung (especially skin, muscle, and nerve) are sampled more often. For example, only 2 of 39 biopsies in the series reported by Chumbley et al4 and 2 of 25 in the series by Guillevin et al5 were taken from lung. The original pathologic description by Churg and Strauss10 was based on the findings in 10 autopsies and 3 skin biopsy specimens. The most detailed histologic description was supplied by Koss et al,11 who reported the findings in 3 lung biopsy specimens and 1 autopsy. A spectrum of histologic changes is found in the lung in Churg-Strauss syndrome Table 1. As in other organs, classically described findings include the combination of tissue infiltration by eosinophils, necrotizing vasculitis, and
768
extravascular granulomas. Tissue eosinophilia is manifested by areas of eosinophilic pneumonia that are characterized by the accumulation within alveolar spaces of large numbers of eosinophils and macrophages accompanied by alveolar septal expansion by a chronic inflammatory cell infiltrate containing numerous eosinophils Image 1 and Image 2. Eosinophilic abscesses may occur, and parenchymal necrosis is sometimes prominent Image 3. Vasculitis typically accompanies the eosinophilic pneumonia and is characterized by intimal and medial infiltration by chronic inflammation containing numerous eosinophils Image 4. It may show granulomatous features or contain numerous giant cells reminiscent of giant cell arteritis Image 5, and fibrinoid necrosis is sometimes present. Necrotizing granulomas are frequently found in the adjacent parenchyma. They may be composed of large foci of necrosis surrounded by a rim of epithelioid histiocytes, or they may consist of small aggregates of epithelioid histiocytes arranged with their long axes perpendicular to the center, much like the spokes of a wheel (so-called palisaded granulomas, Image 6). Although the combination of eosinophilic pneumonia, vasculitis, and granulomatous inflammation is considered diagnostic of Churg-Strauss syndrome, and it was present in the 4 cases described by Koss et al,11 all 3 histologic lesions often are not found together in 1 organ. The original autopsy study reported by Churg and Strauss, 10 for example, described eosinophilic pneumonia-like areas in only one half of the cases, active pulmonary vasculitis in 3 of 10, and extravascular lung granulomas in 2 of 10. Lanham et al2 further emphasized that the 3 histologic components coincided in a given tissue in only 13% of biopsy specimens and 24% of autopsies. These observations underscore the need for careful clinical and pathologic correlation to establish the diagnosis, especially in cases that do not show the full spectrum of histologic changes.
Differential Diagnosis
The histologic differential diagnosis of Churg-Strauss syndrome in the lung includes disorders associated with a prominent eosinophil infiltrate or a combination of
American Society of Clinical Pathologists
Am J Clin Pathol 2000;114:767-772
Anatomic Pathology / REVIEW ARTICLE
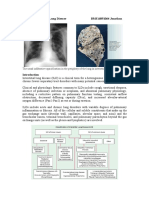
Image 1 Eosinophilic pneumonia in Churg-Strauss syndrome. The alveolar spaces are filled with eosinophils and scattered macrophages, and there is an accompanying proteinaceous exudate. The alveolar septa are mildly thickened by edema and a cellular infiltrate also containing numerous eosinophils (H&E, 225).
Image 2 Higher magnification view of eosinophilic pneumonia showing the large numbers of eosinophils filling an alveolar space associated with edematous and inflamed adjacent alveolar septa (H&E, 480).
Image 3 Irregular parenchymal necrosis in an area of eosinophilic pneumonia in Churg-Strauss syndrome (H&E, 90).
Image 4 Vasculitis in Churg-Strauss syndrome. The walls of this small artery are thickened and distorted by a densely cellular eosinophil infiltrate (H&E, 225).
eosinophils and granulomatous inflammation Table 2. Features helpful in the differential diagnosis of these conditions are summarized in Table 3. Uncomplicated chronic eosinophilic pneumonia is probably the most important lesion to distinguish from ChurgStrauss syndrome, since it is relatively common and usually has less severe clinical implications. It often occurs in people
American Society of Clinical Pathologists
with asthma, and blood eosinophilia may be present, although the degree of eosinophilia is usually not as great as in Churg-Strauss syndrome.13 Eosinophilic pneumonia is characterized by a mixture of eosinophils and macrophages within alveolar spaces associated with a chronic interstitial pneumonia containing numerous eosinophils. Although there may be eosinophil infiltration of blood vessel walls, this
Am J Clin Pathol 2000;114:767-772
769
Katzenstein / CHURG-STRAUSS SYNDROME
Image 5 Granulomatous vasculitis in Churg-Strauss syndrome. The wall of this artery is partially replaced by an infiltrate of epithelioid histiocytes (H&E, 225).
Image 6 Palisaded granuloma in Churg-Strauss syndrome. Epithelioid histiocytes are arranged with their long axis perpendicular to the central zone containing a small focus of necrosis (arrow). Note the adjacent prominent eosinophil infiltrate (H&E, 360).
feature is not prominent, and a necrotizing vasculitis is not present. Necrotizing granulomas, likewise, are not a feature. Collections of necrotic eosinophils may be found within air spaces (so-called eosinophilic abscesses), but the necrosis does not extend into adjacent lung parenchyma. It is possible for biopsy specimens in Churg-Strauss syndrome to show
Table 2 Differential Diagnosis of Churg-Strauss Syndrome in the Lung
Eosinophilic pneumonia (chronic or acute) Hypereosinophilic syndrome Bronchocentric granulomatosis (allergic bronchopulmonary aspergillosis) Wegener granulomatosis Miscellaneous (infections, dirofilarial nodules, eosinophilic vascular infiltration of pneumothorax)
only eosinophilic pneumonia, however, and in those cases, the clinical findings as described earlier are necessary to establish the diagnosis. An acute variant of eosinophilic pneumonia was described in which patients develop respiratory failure. It is characterized by areas of diffuse alveolar damage in addition to the eosinophil infiltration.14 Hypereosinophilic syndrome is a rare condition characterized by persistent blood and bone marrow eosinophilia lasting longer than 6 months associated with evidence of tissue infiltration by eosinophils.15 Heart, skin, nervous system, lungs, gastrointestinal tract, liver, and spleen frequently are involved. The most serious complications result from cardiac or central nervous system dysfunction. Most patients respond to corticosteroid therapy, and hydroxyurea or interferon alfa have been used in refractory cases. Although there may be some overlap with Churg-Strauss
Table 3 Contrasting Features of Churg-Strauss Syndrome and Major Conditions in the Histologic Differential Diagnosis
CSS Blood eosinophilia Asthma Extrapulmonary involvement ANCA Eosinophilic pneumonia Necrotizing vasculitis Necrotizing granuloma Yes, high Yes Yes Yes (P-ANCA in 70%) Yes Yes Yes CEP Occasionally present Occasionally present No No Yes No No HES Yes, high No Yes No No No No BCG Yes Frequently present No No Yes No Yes WG Rare No Yes Yes (60%-90%), usually CANCA No Yes Yes
ANCA, antineutrophil cytoplasmic autoantibody; BCG, bronchocentric granulomatosis; CEP, chronic eosinophilic pneumonia; CSS, Churg-Strauss syndrome; HES, hypereosinophilic syndrome; WG, Wegener granulomatosis.
770
Am J Clin Pathol 2000;114:767-772
American Society of Clinical Pathologists
Anatomic Pathology / REVIEW ARTICLE
syndrome, the absence of asthma clinically and vasculitis and granulomas pathologically distinguishes these 2 conditions. Bronchocentric granulomatosis is a rare disease with an unknown cause in half the cases, but due to allergic bronchopulmonary aspergillosis in the other half.16 In the latter situation, the patients usually have asthma with blood eosinophilia along with systemic symptoms and pulmonary infiltrates. Lung biopsy shows necrotizing granulomatous inflammation that centers on and replaces bronchioles. Eosinophils are usually prominent in the surrounding parenchyma, and areas of eosinophilic pneumonia may be present. The appearance differs from that of Churg-Strauss syndrome because of the exquisitely bronchocentric location of the granulomatous inflammation and by the absence of a necrotizing vasculitis. Also, many patients with bronchocentric granulomatosis have accompanying mucoid impaction of bronchi. This lesion is characterized by dilated bronchi filled with mucin containing lamellated rows of necrotic eosinophils and Charcot-Leyden crystals (so-called allergic mucin) and often also fungal hyphae. Clinically, blood eosinophilia is usually not as high as in Churg-Strauss syndrome, and extrapulmonary involvement is not a feature. Rare cases of Wegener granulomatosis have been described in which tissue eosinophilia is a prominent feature in the lung.17,18 Blood eosinophilia has been present in some cases as well, but none of the patients have had asthma. These cases differ pathologically from Churg-Strauss syndrome in that areas resembling eosinophilic pneumonia are not present. Rather, the eosinophil infiltrate is present within and around the granulomatous inflammation. In difficult cases, however, clinical and laboratory findings should help make the distinction, although rare patients with overlapping features of both diseases have been described.19 A number of other conditions may be associated with tissue eosinophilia and granulomatous inflammation and, thus, may enter the differential diagnosis of Churg-Strauss syndrome. Infections are the most important, and coccidioidomycosis , especially, is known to cause this reaction.20,21 A careful search for organisms by using special stains is warranted in all cases and should obviate the problem. Dirofilarial nodules often resemble necrotizing granulomas and may have a surrounding prominent eosinophil infiltrate.22 They are usually solitary nodular lesions, however, and although vascular inflammation may be present, a necrotizing vasculitis does not occur. In addition, the worm should be easily visible within blood vessel lumens in the necrotic zones. A perivascular and interstitial infiltrate of eosinophils has been described in patients with pneumothorax and presumably is related to chest tube placement.23 It is an isolated finding without granulomatous inflammation in a clinical situation that has no features suggestive of ChurgStrauss syndrome. Eosinophils are often prominent in cases
American Society of Clinical Pathologists
of eosinophilic granuloma, but diagnosis should not be difficult, since Langerhans cells constitute the main cellular infiltrate, and true granulomatous inflammation is absent.
From Crouse Hospital and Upstate Medical University, Syracuse, NY. Address reprint requests to Dr Katzenstein: 736 Irving Ave, Syracuse, NY 13210.
References
1. Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: proposal of an International Consensus Conference. Arthritis Rheum. 1994;37:187-192. 2. Lanham JG, Elkon KB, Pusey CD, et al. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine. 1984;63:65-81. 3. Lhote F, Guillevin L. Polyarteritis nodosa, microscopic polyangiitis, and Churg-Strauss syndrome: clinical aspects and treatment. Rheum Dis Clin North Am. 1995;21:911-947. 4. Chumbley LC, Harrison EG Jr, DeRemee RA. Allergic granulomatosis and angiitis (Churg-Strauss syndrome): report and analysis of 30 cases. Mayo Clin Proc. 1977;52:477-484. 5. Guillevin L, Guittard T, Bletry O, et al. Systemic necrotizing angiitis with asthma: causes and precipitating factors in 43 cases. Lung. 1987;165:165-172. 6. Wechsler ME, Finn D, Gunawardena D, et al. Churg-Strauss syndrome in patients receiving montelukast as treatment for asthma. Chest. 2000;117:708-713. 7. Choi YH, Im J-G, Han BK, et al. Thoracic manifestation of Churg-Strauss syndrome: radiologic and clinical findings. Chest. 2000;117:117-124. 8. Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512-1523. 9. Buschman DL, Waldron JA Jr, King TE Jr. Churg-Strauss pulmonary vasculitis: high-resolution computed tomography scanning and pathologic findings. Am Rev Respir Dis. 1990;142:458-461. 10. Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol. 1951;277-294. 11. Koss MN, Antonovych T, Hochholzer L. Allergic granulomatosis (Churg-Strauss syndrome): pulmonary and renal morphologic findings. Am J Surg Pathol. 1981;5:21-28. 12. Wishnick MM, Valensi Q, Doyle EF, et al. Churg-Strauss syndrome: development of cardiomyopathy during corticosteroid treatment. Am J Dis Child. 1982;136:339-344. 13. Allen JN, Davis WB. Eosinophilic lung diseases. Am J Respir Crit Care Med. 1994;150(5 pt 1):1423-1438. 14. Tazelaar HD, Linz LJ, Colby TV, et al. Acute eosinophilic pneumonia: histopathologic findings in nine patients. Am J Respir Crit Care Med. 1997;155:296-302. 15. Weller PF, Bubley GJ. The idiopathic hypereosinophilic syndrome. Blood. 1994;83:2759-2779. 16. Bosken CH, Myers JL, Greenberger PA, et al. Pathologic features of allergic bronchopulmonary aspergillosis. Am J Surg Pathol. 1988;12:216-222. 17. Potter MB, Fincher RK, Finger DR. Eosinophilia in Wegeners granulomatosis. Chest. 1999;116:1480-1483. 18. Yousem SA, Lombard CM. Eosinophilic variant of Wegeners granulomatosis. Hum Pathol. 1988;19:682-688.
Am J Clin Pathol 2000;114:767-772
771
Katzenstein / CHURG-STRAUSS SYNDROME
19. Yousem SA, Hochholzer L. Overlap syndromes: Wegeners granulomatosis and Churg-Strauss syndrome. Semin Respir Med. 1989;10:162-166. 20. Lombard CM, Tazelaar HD, Krasne DL. Pulmonary eosinophilia in coccidioidal infections. Chest. 1987;91:734736. 21. Schermoly MJ, Hinthorn DR. Eosinophilia in coccidioidomycosis. Arch Intern Med. 1988;148:895-896.
22. Katzenstein A-LA. Katzenstein and Askins Surgical Pathology of Non-Neoplastic Lung Disease: Major Problems in Pathology. Vol 13. 3rd ed. Philadelphia, PA: Saunders; 1997:305-307. 23. Luna E, Tomashefski JF Jr, Brown D, et al. Reactive eosinophilic pulmonary vascular infiltration in patients with spontaneous pneumothorax. Am J Surg Pathol. 1994;18:195199.
772
Am J Clin Pathol 2000;114:767-772
American Society of Clinical Pathologists
You might also like
- Perm State Medical University Churg-Strauss Syndrome: - Joisy Aloor 5 YearDocument33 pagesPerm State Medical University Churg-Strauss Syndrome: - Joisy Aloor 5 YearOlga GoryachevaNo ratings yet
- Churg-Strauss Syndrome: Dispelling The MythsDocument18 pagesChurg-Strauss Syndrome: Dispelling The MythsJoss VaNo ratings yet
- Jurnal HidroponikDocument6 pagesJurnal HidroponiknurfadillahmzNo ratings yet
- Fisiologi Sistem Saraf TepiDocument19 pagesFisiologi Sistem Saraf TepiNata NayottamaNo ratings yet
- ChurgDocument2 pagesChurgdian desiaNo ratings yet
- Pulmonary EmbolismDocument25 pagesPulmonary EmbolismRaymund VadilNo ratings yet
- Dr. Sana Bashir DPT, MS-CPPTDocument46 pagesDr. Sana Bashir DPT, MS-CPPTbkdfiesefll100% (1)
- Pulmonary EosinophiliaDocument19 pagesPulmonary EosinophiliaOlga GoryachevaNo ratings yet
- Esti2012 E-0066Document40 pagesEsti2012 E-0066nistor97No ratings yet
- Tension PneumothoraxDocument40 pagesTension Pneumothoraxbemi lestariNo ratings yet
- Lung AbscessDocument4 pagesLung AbscessDholla Zulmitra100% (1)
- Patrones Tomograficos Epid PDFDocument28 pagesPatrones Tomograficos Epid PDFYony Morales LeonNo ratings yet
- 2010 Hemorragia Alveolar DifusaDocument8 pages2010 Hemorragia Alveolar DifusaCecilia Requejo TelloNo ratings yet
- 05 Eosinophilic PneumoniasDocument33 pages05 Eosinophilic PneumoniasAhmad TwenyNo ratings yet
- SDL 18 Interstitial Lung Disease BMS16091064Document8 pagesSDL 18 Interstitial Lung Disease BMS16091064Jonathan YeohNo ratings yet
- The Management of Subcutaneous Emphysema in PneumoDocument7 pagesThe Management of Subcutaneous Emphysema in PneumoRobert ChristevenNo ratings yet
- A 56 Year Old Woman With Multiple Pulmonary CystsDocument8 pagesA 56 Year Old Woman With Multiple Pulmonary CystsAchmad Dodi MeidiantoNo ratings yet
- Understanding Bronchiectasis: Causes, Symptoms and TreatmentDocument60 pagesUnderstanding Bronchiectasis: Causes, Symptoms and TreatmentArulNo ratings yet
- Respiratory Chest PainDocument16 pagesRespiratory Chest Painwinardi rNo ratings yet
- Clinical Presentation and Diagnosis of PneumothoraxDocument49 pagesClinical Presentation and Diagnosis of PneumothoraxisahNo ratings yet
- Bookshelf NBK553857Document12 pagesBookshelf NBK553857Sanda Škrinjarić-CincarNo ratings yet
- Asthmatic Granulomatosis 2012Document7 pagesAsthmatic Granulomatosis 2012Edoardo CavigliNo ratings yet
- Thorax00193 0009 PDFDocument10 pagesThorax00193 0009 PDFhotmart ventasNo ratings yet
- Jurnal 2Document0 pagesJurnal 2Seftiana SaftariNo ratings yet
- Metod Terapiya Lechebnoe Delo Fakul'tet Inostranyx Studentov-006Document29 pagesMetod Terapiya Lechebnoe Delo Fakul'tet Inostranyx Studentov-006Nishani SatiyaseelanNo ratings yet
- Dapus 3Document10 pagesDapus 3Yan Agus AchtiarNo ratings yet
- Final Written Report 4)Document13 pagesFinal Written Report 4)chocoholic potchiNo ratings yet
- CompilationDocument24 pagesCompilationKath RubioNo ratings yet
- Literature Review 3 TopicDocument27 pagesLiterature Review 3 TopicBharathi Sneha PeriasamyNo ratings yet
- Angiolymphoid Hyperplasia With Eosinophilia in The Lungs: A Complex Name For An Innocuous Disease?Document2 pagesAngiolymphoid Hyperplasia With Eosinophilia in The Lungs: A Complex Name For An Innocuous Disease?Sango AyraNo ratings yet
- Differential Diagnosis of TBC Pleurisy.Document13 pagesDifferential Diagnosis of TBC Pleurisy.Yvonne Nmeli MihesNo ratings yet
- The Management of Subcutaneous Emphysema in Pneumothorax: A Literature ReviewDocument6 pagesThe Management of Subcutaneous Emphysema in Pneumothorax: A Literature ReviewVivian FatharaniNo ratings yet
- ABIGAIL 2010_Diffuse Alveolar HemorrhageDocument10 pagesABIGAIL 2010_Diffuse Alveolar HemorrhageRafael JustinoNo ratings yet
- How Does COPD Differ From AsthmaDocument2 pagesHow Does COPD Differ From AsthmaRenz AducaNo ratings yet
- Case Report: Pulmonary Sequestration: A Case Report and Literature ReviewDocument4 pagesCase Report: Pulmonary Sequestration: A Case Report and Literature Reviewjo_jo_maniaNo ratings yet
- Trapped Lung - StatPearls - NCBI BookshelfDocument7 pagesTrapped Lung - StatPearls - NCBI BookshelfVinna KusumawatiNo ratings yet
- Pneumothorax Classification and Etiology: Clinics in Chest Medicine November 2021Document18 pagesPneumothorax Classification and Etiology: Clinics in Chest Medicine November 2021DesyaNo ratings yet
- Systemic: Manifestations Erythema NodosumDocument6 pagesSystemic: Manifestations Erythema NodosumMuhammad ZubaidiNo ratings yet
- PDF To WordDocument17 pagesPDF To Wordanisa ghinaNo ratings yet
- Bronquiolite DiagnosticoDocument14 pagesBronquiolite DiagnosticoFrederico PóvoaNo ratings yet
- Temporal Bone GranulomaDocument5 pagesTemporal Bone GranulomaRabie MohamedNo ratings yet
- Surgical Treatment of Pulmonary AspergillomaDocument6 pagesSurgical Treatment of Pulmonary AspergillomarynaldiandriansyaNo ratings yet
- Chronic Necrotising Pulmonary Aspergillosis - Radiology Reference Article - RadiopaediaDocument3 pagesChronic Necrotising Pulmonary Aspergillosis - Radiology Reference Article - RadiopaediaNurfatriani DardinNo ratings yet
- COPD and Pulmonary Thromboembolism (For Galley Proof)Document6 pagesCOPD and Pulmonary Thromboembolism (For Galley Proof)Ram AdhikariNo ratings yet
- ThoraxDocument56 pagesThoraxpriskilas911No ratings yet
- Lung AbcessDocument11 pagesLung AbcessGul SapiNo ratings yet
- Massive Hemoptysis: Resident Grand RoundsDocument7 pagesMassive Hemoptysis: Resident Grand RoundsEko CahyonoNo ratings yet
- Diagnosing Bronchiectasis: An Overlooked DiseaseDocument6 pagesDiagnosing Bronchiectasis: An Overlooked DiseasefallenczarNo ratings yet
- SarcoidosisDocument59 pagesSarcoidosisMaria Dodon100% (2)
- Pneumothorax Classification and Etiology: Clinics in Chest Medicine November 2021Document18 pagesPneumothorax Classification and Etiology: Clinics in Chest Medicine November 2021Maria Asri PranataNo ratings yet
- "Imitators" of The ARDS Implications For Diagnosis and Treatment Acute Lung Injury (ALI) and ARDS (ALI/ARDS)Document22 pages"Imitators" of The ARDS Implications For Diagnosis and Treatment Acute Lung Injury (ALI) and ARDS (ALI/ARDS)Odett EzzatNo ratings yet
- COP - Cryptogenic Organizing Pneumonia - StatPearls - NCBI BookshelfDocument7 pagesCOP - Cryptogenic Organizing Pneumonia - StatPearls - NCBI Bookshelfrbatjun576No ratings yet
- Pneumothorax Classification and Etiology: Clinics in Chest Medicine November 2021Document18 pagesPneumothorax Classification and Etiology: Clinics in Chest Medicine November 2021joaquinNo ratings yet
- Assessing Dyspnea: History and Exam Guide EvaluationDocument2 pagesAssessing Dyspnea: History and Exam Guide EvaluationCarDi Tran NhanNo ratings yet
- Pneumothorax: Classification and EtiologyDocument17 pagesPneumothorax: Classification and EtiologyPASMOXNo ratings yet
- Interstitial Lung DiseaseDocument4 pagesInterstitial Lung DiseasedewimarisNo ratings yet
- Congenital Lobar EmphysemaDocument3 pagesCongenital Lobar Emphysemamadimadi11No ratings yet
- The Unusual SuspectDocument8 pagesThe Unusual Suspecteduardokenedy2No ratings yet
- Journal of Thoracic ImagingDocument11 pagesJournal of Thoracic Imagingestues2No ratings yet
- Respiratory Step 2 CK NoteDocument41 pagesRespiratory Step 2 CK NoteXboyx MahdiNo ratings yet
- Community-Acquired Pneumonia Requiring Hospitalization Among U.S. ChildrenDocument11 pagesCommunity-Acquired Pneumonia Requiring Hospitalization Among U.S. ChildrenHanida Rahmah TaminNo ratings yet
- 1adrenals ESWDocument65 pages1adrenals ESWMuchamad ZubaidNo ratings yet
- Clinical Practice Guidelines For The Management of HypertensionDocument13 pagesClinical Practice Guidelines For The Management of HypertensionDaniel Opazo DamianiNo ratings yet
- WPC 117089Document40 pagesWPC 117089leonjunchan_66965707No ratings yet
- JNC 8Document14 pagesJNC 8amiwahyuniNo ratings yet
- Guidelines Management 2007w Bookmarks PDFDocument0 pagesGuidelines Management 2007w Bookmarks PDFdiaga081No ratings yet
- Prognosis GuidelinesDocument103 pagesPrognosis GuidelinesPaola MedinaNo ratings yet
- Vasculitis, Churg Strauss SyndromeDocument7 pagesVasculitis, Churg Strauss SyndromeMuchamad ZubaidNo ratings yet
- Churg Strauss SyndromeDocument8 pagesChurg Strauss SyndromeMuchamad ZubaidNo ratings yet
- Prognosis GuidelinesDocument103 pagesPrognosis GuidelinesPaola MedinaNo ratings yet
- Vasculitis, Churg Strauss SyndromeDocument25 pagesVasculitis, Churg Strauss SyndromeMuchamad ZubaidNo ratings yet
- Churg Strauss SyndromeDocument8 pagesChurg Strauss SyndromeMuchamad ZubaidNo ratings yet
- Acute PainDocument3 pagesAcute PainGerardeanne ReposarNo ratings yet
- BPC-157 A Collection of StudiesDocument28 pagesBPC-157 A Collection of StudiesscribdNo ratings yet
- Philcare Application Form - 07302013Document6 pagesPhilcare Application Form - 07302013Jerry Barad SarioNo ratings yet
- PSYCH 1.1A Schizophrenia Spectrum and Other Psychotic DisorderDocument28 pagesPSYCH 1.1A Schizophrenia Spectrum and Other Psychotic DisorderZaza100% (1)
- UTI in ChildrenDocument94 pagesUTI in ChildrenSarfraz AnsariNo ratings yet
- About Lung Cancer: Overview and TypesDocument13 pagesAbout Lung Cancer: Overview and TypesGordana PuzovicNo ratings yet
- Psychiatry Passmedicine & Onexamination Notes 2016 PDFDocument34 pagesPsychiatry Passmedicine & Onexamination Notes 2016 PDFJyothi ReddyNo ratings yet
- Atrophic Pap Smears, Differential Diagnosis and Pitfalls: A ReviewDocument5 pagesAtrophic Pap Smears, Differential Diagnosis and Pitfalls: A ReviewJill ValdesNo ratings yet
- Lab Blood TypesDocument3 pagesLab Blood TypesTrizia M MendezNo ratings yet
- Nursing Care PlanDocument2 pagesNursing Care PlanDan TijamNo ratings yet
- Basic First Aid Procedures for EmergenciesDocument4 pagesBasic First Aid Procedures for EmergenciesSoyPedroNo ratings yet
- Castro Et Al. ProtocoloDocument10 pagesCastro Et Al. ProtocoloFrancisco Alfonso Burgos JuliánNo ratings yet
- Diagnosing rare facial tumorsDocument1 pageDiagnosing rare facial tumorsTayyaba RafiqNo ratings yet
- Trematode SDocument26 pagesTrematode SothnielNo ratings yet
- HHFB Endowment Tri-FoldDocument2 pagesHHFB Endowment Tri-FoldStephanie Martin HarmonNo ratings yet
- Who Dehidrasi PG 17 PDFDocument52 pagesWho Dehidrasi PG 17 PDFSasha ManoNo ratings yet
- Neha ShahDocument448 pagesNeha ShahAshima GautamNo ratings yet
- Methamphetamine Addiction: A Review of The LiteratureDocument8 pagesMethamphetamine Addiction: A Review of The Literaturesaghita fauziahNo ratings yet
- Rapid Review of Hematology (Aug 31, 2013) - (9350909618) - (Jaypee Brothers Medical Pub)Document149 pagesRapid Review of Hematology (Aug 31, 2013) - (9350909618) - (Jaypee Brothers Medical Pub)Dana AhmedNo ratings yet
- Test Report: Patient ID 0100007634 Sid NoDocument2 pagesTest Report: Patient ID 0100007634 Sid NoBalamurugan ArumugamNo ratings yet
- Pediatric OSCE A Guide For Medical StudentsDocument9 pagesPediatric OSCE A Guide For Medical Studentsjoey92leeNo ratings yet
- Femur fracture assessment and treatment exam questionsDocument8 pagesFemur fracture assessment and treatment exam questionsKwabena Amankwa100% (2)
- CopdDocument60 pagesCopdRizqy Shofianingrum100% (1)
- 2 Oral UlcersDocument27 pages2 Oral UlcersAhmed Abdelhady Mahmoud AbdelwahedNo ratings yet
- SLU Pathology CBL Cases for Gastrointestinal and Liver PathologyDocument6 pagesSLU Pathology CBL Cases for Gastrointestinal and Liver PathologyAaron O. CafeNo ratings yet
- Labor and ComplicationsDocument51 pagesLabor and ComplicationsTrisha Mae MarquezNo ratings yet
- Old Canalicular Laceration Repair A Retrospective Study ofDocument6 pagesOld Canalicular Laceration Repair A Retrospective Study ofMuh Syarifullah ANo ratings yet
- Disaster Associated Health IssuesDocument29 pagesDisaster Associated Health Issuesmeshack mbalaNo ratings yet
- Research WorkDocument50 pagesResearch WorkSudhanshu SaxenaNo ratings yet
- Congenital Scoliosis Case Study: Ashley (6-7 YearsDocument20 pagesCongenital Scoliosis Case Study: Ashley (6-7 YearsIulia Dulgheru100% (1)