Professional Documents
Culture Documents
Balz Schiemann Reaction PDF
Balz Schiemann Reaction PDF
Uploaded by
Matanda Katumba0 ratings0% found this document useful (0 votes)
88 views1 pageThe Balz–Schiemann reaction transforms anilines to aryl fluorides via diazonium fluoroborates. The aromatic primary amine is first diazotized with sodium nitrite in the presence of fluoroboric acid at low temperatures to form an aryl diazonium tetrafluroborate. Upon heating, this intermediate decomposes to yield the corresponding aryl fluoride. The Balz–Schiemann reaction provides a preferred route for producing fluorobenzene compared to direct fluorination, which is a more violent reaction that is difficult to control.
Original Description:
Original Title
Balz Schiemann Reaction.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe Balz–Schiemann reaction transforms anilines to aryl fluorides via diazonium fluoroborates. The aromatic primary amine is first diazotized with sodium nitrite in the presence of fluoroboric acid at low temperatures to form an aryl diazonium tetrafluroborate. Upon heating, this intermediate decomposes to yield the corresponding aryl fluoride. The Balz–Schiemann reaction provides a preferred route for producing fluorobenzene compared to direct fluorination, which is a more violent reaction that is difficult to control.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
88 views1 pageBalz Schiemann Reaction PDF
Balz Schiemann Reaction PDF
Uploaded by
Matanda KatumbaThe Balz–Schiemann reaction transforms anilines to aryl fluorides via diazonium fluoroborates. The aromatic primary amine is first diazotized with sodium nitrite in the presence of fluoroboric acid at low temperatures to form an aryl diazonium tetrafluroborate. Upon heating, this intermediate decomposes to yield the corresponding aryl fluoride. The Balz–Schiemann reaction provides a preferred route for producing fluorobenzene compared to direct fluorination, which is a more violent reaction that is difficult to control.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
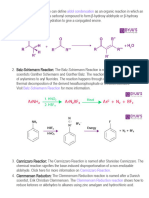
Mechanism of BalzSchiemann Reaction
BalzSchiemann Reaction
The Schiemann reaction (also called the BalzSchiemann reaction) is a chemical reaction in
which anilines are transformed to aryl fluorides via diazonium fluoroborates . Named after the
German chemists Gnther Schiemann and Gnther Balz, this reaction is the preferred route to
fluorobenzene. acid.
Fluoroarenes (aryl fluorides ) cannot be prepared by the direct fluorination of aromatic
hydrocarbon ,since the reaction is very violent and cannot be easily controlled. These can
however be easily prepared by BalzSchiemann reaction. in this reaction the aromatic primary
amine is first diazotized with NaNO2 in the presence of HBF4 (Fluoroboric acid) at 273-278 K
and the aryl diazonium tetrafluroborate yhus formed is heated to give the corresponding aryl
fluoride.
Mechanism
CHEMISTRY tUTORIALS III A 41 Nehru Nagar, Ghaziabad. U.P. Pin 201001
www.chemistrytutorial.in email:- chtgzb@gmail.com ,
Mobile:-7042090378,7579218698
You might also like
- 30 Important Name Reactions Organic Chemistry For IIT JEEDocument6 pages30 Important Name Reactions Organic Chemistry For IIT JEEYo33% (3)
- 06 Chapter 1Document63 pages06 Chapter 1Kautsar NurfalaqNo ratings yet
- Balz Schiemann Reaction PDFDocument1 pageBalz Schiemann Reaction PDFyashNo ratings yet
- (@aakash - Test - PDFS) All Name Reaction One ShotDocument101 pages(@aakash - Test - PDFS) All Name Reaction One ShotSohil SharmaNo ratings yet
- M Sc-1Document12 pagesM Sc-1Shreyas BhandaryNo ratings yet
- AMINESDocument4 pagesAMINESMudit SharmaNo ratings yet
- AMINESDocument4 pagesAMINESmuhammadNo ratings yet
- Passing Package, Haloalkanes and HaloarenesDocument8 pagesPassing Package, Haloalkanes and HaloarenesShalem JeldiNo ratings yet
- Important Name ReactionsDocument10 pagesImportant Name Reactionsbibek64m5No ratings yet
- Organic Chemistry Important Reagents-EditDocument19 pagesOrganic Chemistry Important Reagents-Edit10C 27 SAI VISHWA JETHNo ratings yet
- Chemistry HSC Board Important Reactions PDFDocument47 pagesChemistry HSC Board Important Reactions PDFNutzie YNo ratings yet
- Name Reactions1Document4 pagesName Reactions1Vijaya RajuNo ratings yet
- The Most Important Part of ChemistryDocument10 pagesThe Most Important Part of ChemistryPreetam PaulNo ratings yet
- Organic Chemistry Name ReactionDocument13 pagesOrganic Chemistry Name ReactionDeep GuptaNo ratings yet
- Nucleophilic Aromatic Substitution: From Wikipedia, The Free EncyclopediaDocument4 pagesNucleophilic Aromatic Substitution: From Wikipedia, The Free EncyclopediasantiisantNo ratings yet
- Naming Reactions Organic ChemistryDocument8 pagesNaming Reactions Organic Chemistrysaapldesign1 1No ratings yet
- Reacciones Con FosforoDocument8 pagesReacciones Con FosforoRicardo DoroteoNo ratings yet
- Poc Unit-5Document9 pagesPoc Unit-5Bintoo SharmaNo ratings yet
- Chapter 2Document15 pagesChapter 2Hongxi Zhang100% (1)
- GonzalezandHalloranreactionphosphoricacidaluminaBulletin Vol-59 No-07 July 1980-2Document7 pagesGonzalezandHalloranreactionphosphoricacidaluminaBulletin Vol-59 No-07 July 1980-2SunnyNo ratings yet
- Chap 16 (Notes)Document134 pagesChap 16 (Notes)Steffi YapNo ratings yet
- Reactions of Synthetic ImportanceDocument28 pagesReactions of Synthetic ImportanceRx Nadeem ChhipaNo ratings yet
- Hsslive-Xii-Chem-10. Alkyhalides and Aryl HalidesDocument13 pagesHsslive-Xii-Chem-10. Alkyhalides and Aryl HalidesHakim AbbasNo ratings yet
- Shapiro Reaction and BamfordDocument13 pagesShapiro Reaction and BamfordharishNo ratings yet
- Organic Chemistry Named Reaction InDetail by MeritnationDocument9 pagesOrganic Chemistry Named Reaction InDetail by MeritnationAtif AteeqNo ratings yet
- Reactions of Aromatic AminesDocument21 pagesReactions of Aromatic Aminessayyed mohsinaNo ratings yet
- Alkyl HalidesDocument20 pagesAlkyl HalidesShivam Gupta0% (1)
- Name ReactionsDocument10 pagesName ReactionsParam SoniNo ratings yet
- 12-CBSE Name ReactionsDocument10 pages12-CBSE Name Reactionsdasunnayan4No ratings yet
- Aryl HalidesDocument23 pagesAryl HalidesSteveNo ratings yet
- Organic Named File SarwarDocument8 pagesOrganic Named File SarwarShohom DeNo ratings yet
- AMINES XiiDocument17 pagesAMINES XiiZONE OF CUBENo ratings yet
- Name ReacitonsDocument6 pagesName ReacitonspzohmingthangaNo ratings yet
- Subject ChemistryDocument13 pagesSubject Chemistryana pertiwiNo ratings yet
- 22.4: Alpha Bromination of Carboxylic Acids: ObjectivesDocument3 pages22.4: Alpha Bromination of Carboxylic Acids: ObjectivesTinotenda ZisengweNo ratings yet
- Experiment 2: Haloalkanes: Reaction of HaloalkanesDocument6 pagesExperiment 2: Haloalkanes: Reaction of HaloalkanesEssay NationNo ratings yet
- Anims and UreaDocument25 pagesAnims and UreaShivam GuptaNo ratings yet
- Rightpdf - Hsslive-Xii-Chem-13. B - WatermarkDocument10 pagesRightpdf - Hsslive-Xii-Chem-13. B - Watermarkaksa.bonvoyageNo ratings yet
- Important Chemical Reactions For Class 12 ChemistryDocument10 pagesImportant Chemical Reactions For Class 12 ChemistryRitishNo ratings yet
- L2 - Aromatic Electrophilic Substitution PDFDocument32 pagesL2 - Aromatic Electrophilic Substitution PDFPadamNo ratings yet
- (L2) - (JLD 3.0) - Haloalkane & Haloarenes - 08 SeptDocument49 pages(L2) - (JLD 3.0) - Haloalkane & Haloarenes - 08 Septfunnyvideos. comNo ratings yet
- Important Name ReactionsDocument62 pagesImportant Name ReactionsMahi SharmaNo ratings yet
- Trinitrotoluene (TNT), A Pale Yellow, Solid Organic Nitrogen Compound Used Chiefly As An Explosive, Prepared by Stepwise Nitration of TolueneDocument76 pagesTrinitrotoluene (TNT), A Pale Yellow, Solid Organic Nitrogen Compound Used Chiefly As An Explosive, Prepared by Stepwise Nitration of TolueneVidhan PatniNo ratings yet
- ARENEDocument16 pagesARENEkaslana kianaNo ratings yet
- BP401T 5Document18 pagesBP401T 5saheedvkNo ratings yet
- My AssignmentDocument13 pagesMy AssignmentAmos JamesNo ratings yet
- Class12 - Organichemistry - Named ReactionsDocument6 pagesClass12 - Organichemistry - Named ReactionsMᴀïᴢᴍɛɛŋ AŋꜱᴀʀïNo ratings yet
- Unit ProcessDocument38 pagesUnit ProcessHaris WaqarNo ratings yet
- Jphysiol 1897 sp000678Document7 pagesJphysiol 1897 sp000678Isam SalehNo ratings yet
- C2B Electrophilic Aromatic Substitution Reactions 2019Document59 pagesC2B Electrophilic Aromatic Substitution Reactions 2019Mandisa Mandy SitolotoloNo ratings yet
- Chemsitry Important Revision Notes For CBSE Class 12 Chapter 10Document20 pagesChemsitry Important Revision Notes For CBSE Class 12 Chapter 10himanshuchoudhary8534No ratings yet
- Vollhardt 6e Lecture PowerPoints - Chapter 12Document74 pagesVollhardt 6e Lecture PowerPoints - Chapter 12superfr3shmNo ratings yet
- Updated Grade 12 Organic Name Reactions-Units 10,11,12,13Document10 pagesUpdated Grade 12 Organic Name Reactions-Units 10,11,12,13Tanmay SharmaNo ratings yet
- Organic Chemistry-Named ReactionsDocument10 pagesOrganic Chemistry-Named Reactionsatharvbaghel4444No ratings yet
- Amines S-3Document8 pagesAmines S-3sciencewing rbiNo ratings yet
- A Further Investigation of the Symmetrical Chloride of Paranitroorthosulphobenzoic AcidFrom EverandA Further Investigation of the Symmetrical Chloride of Paranitroorthosulphobenzoic AcidNo ratings yet
- Boron Fluoride and Its Compounds as Catalysts in Organic Chemistry: International Series of Monographs on Organic ChemistryFrom EverandBoron Fluoride and Its Compounds as Catalysts in Organic Chemistry: International Series of Monographs on Organic ChemistryNo ratings yet