Professional Documents
Culture Documents
Macroscopic Electrostatics
Uploaded by
EddieOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Macroscopic Electrostatics
Uploaded by
EddieCopyright:
Available Formats
88 CHAPTER 4 .
MACROSCOPIC ELECTROSTATICS
we obtain
(E(r))= -V ds/ c[ 1
r-slV v
dV
j
qjkb(rj - s - r)
t)S,
+ (V, r - s l dV
j
pjS(rj - s - r)] (4.5)
(interchanging averaging and taking of gradient). We now set
where we define as
N ( s ) : the number of molecules per unit volume at s ,
(qmol(S)): average charge per molecule at s ,
p ( s ) : macroscopic charge density at s ,
P(s): polarisation vector (= dipole m o m e n t per unit volume) at s ,
(Pmol(s)): average dipole m o m e n t per molecule at s.
We then have (with s replaced by r)
E*(r)= (E(r))= -V + kP(r) . v-Ir - rl
E
S, [
-V& vq5p. -
dV -
In view of continuity for r # r it is permissible to write the divergence (see
explanations below)
1
kp(r)V2-
Ir - rl
kp(r)b(r - r) + kP(r) . V6(r - r)
= 47rkp(r) - 47rkV P(r)
1
(4.7)
where in the step from the first line to the second we used the replacement
1
V2---+ -47r6(r - r),
Ir - rl
You might also like
- 88 PDFDocument1 page88 PDFEddieNo ratings yet
- 88 PDFDocument1 page88 PDFEddieNo ratings yet
- Electrostatics: 3.1 The Electric FieldDocument8 pagesElectrostatics: 3.1 The Electric FieldirinaNo ratings yet
- Electrostatic FieldsDocument40 pagesElectrostatic FieldscharanNo ratings yet
- Field Theory Concepts-Edit Part8Document10 pagesField Theory Concepts-Edit Part8teddyNo ratings yet
- Spoor Veldpaus 1980 Rigid Body Motion Calculated From Spatial Coordinates of MarkersDocument3 pagesSpoor Veldpaus 1980 Rigid Body Motion Calculated From Spatial Coordinates of Markersdanasolav1798No ratings yet
- WKB Method for Solving Dirac EquationDocument6 pagesWKB Method for Solving Dirac EquationVdhieieNo ratings yet
- Dirac and Schrödinger equations solved for inverted generalized hyperbolic potentialDocument9 pagesDirac and Schrödinger equations solved for inverted generalized hyperbolic potentialOsarodion EbomwonyiNo ratings yet
- Foundations of Electrodynamics ( (IC221) ) : Instructor: Suman Kalyan Pal (SKP)Document19 pagesFoundations of Electrodynamics ( (IC221) ) : Instructor: Suman Kalyan Pal (SKP)Vishal MaharNo ratings yet
- Error Analysis of Direct Methods of Matrix InversionDocument50 pagesError Analysis of Direct Methods of Matrix Inversionagbas20026896No ratings yet
- Space Flight QuizDocument2 pagesSpace Flight QuizbooksforfunNo ratings yet
- Radial Schrödinger and Dirac wave equations solverDocument44 pagesRadial Schrödinger and Dirac wave equations solverYina BarmanNo ratings yet
- Electrostatic Fields ExplainedDocument28 pagesElectrostatic Fields ExplainedzaidNo ratings yet
- MIT Physics Lecture Notes on Conductors and CapacitanceDocument6 pagesMIT Physics Lecture Notes on Conductors and CapacitanceJefersonNo ratings yet
- 18 Final Exercises and Problems in CalculusDocument34 pages18 Final Exercises and Problems in Calculuslucia lopez lopezNo ratings yet
- L5 PostDocument23 pagesL5 Postvivianzhao010No ratings yet
- Sedimentation Fundamentals and DefinitionsDocument10 pagesSedimentation Fundamentals and DefinitionsPocolee CarterNo ratings yet
- Fundamentals of Condensed Matter Physics (PDFDrive) (161-208)Document48 pagesFundamentals of Condensed Matter Physics (PDFDrive) (161-208)ehab adnanNo ratings yet
- Field Theory Concepts-Edit Part7Document10 pagesField Theory Concepts-Edit Part7teddyNo ratings yet
- EE 351 Electromagnetic Fields LectureDocument20 pagesEE 351 Electromagnetic Fields Lectureomarsiddiqui8No ratings yet
- Formulas 1 PDFDocument18 pagesFormulas 1 PDFBenci MonoriNo ratings yet
- Lecture 14 2019 Potential of Mean Force FinalDocument8 pagesLecture 14 2019 Potential of Mean Force FinalJay SteeleNo ratings yet
- Emfesoln chp08Document26 pagesEmfesoln chp08Mei RatnaaNo ratings yet
- 8.06 Antisymmetric Wavefunctions Can Be Represented by Slater DeterminantsDocument4 pages8.06 Antisymmetric Wavefunctions Can Be Represented by Slater DeterminantsPedroNo ratings yet
- Relativistic Transformations of Electromagnetic IntegralsDocument16 pagesRelativistic Transformations of Electromagnetic IntegralsFriny FernándezNo ratings yet
- The One-Filter Keefe Clarinet ToneholeDocument4 pagesThe One-Filter Keefe Clarinet ToneholeSeminario de Clarinete100% (1)
- PH1020 Assignmnet2-SolutionDocument7 pagesPH1020 Assignmnet2-SolutionJaanav Mathavan me22b007No ratings yet
- Fast Solution of Volume-Surface Integral Equations For Conducting-Dielectric StructuresDocument4 pagesFast Solution of Volume-Surface Integral Equations For Conducting-Dielectric StructuresAfrican LOVENo ratings yet
- 02 Tutorial Vectors+ElectrostaticsDocument2 pages02 Tutorial Vectors+ElectrostaticsmukeshNo ratings yet
- Riesz Fractional Derivatives and Fractional Dimensional SpaceDocument6 pagesRiesz Fractional Derivatives and Fractional Dimensional SpaceHugo Andrade AraújoNo ratings yet
- 1 4πϵ න - റr − റr′ - න റJ (റr′) - റr − റ𝑟′ - Poisson's equations SolutionsDocument8 pages1 4πϵ න - റr − റr′ - න റJ (റr′) - റr − റ𝑟′ - Poisson's equations Solutions鄒雨笙 TZOU,YU-SHENG F64081070No ratings yet
- 2023 EM1 hw3Document3 pages2023 EM1 hw3810003No ratings yet
- Theoretical Physics Multicenter ScatteringDocument8 pagesTheoretical Physics Multicenter ScatteringDanielAlejandroBonillaMorenoNo ratings yet
- Spacecraft Dynamics Homework 1Document6 pagesSpacecraft Dynamics Homework 1TrevorNo ratings yet
- Poisson's equations solutions retarded potentials plane wavesDocument16 pagesPoisson's equations solutions retarded potentials plane waves鄒雨笙 TZOU,YU-SHENG F64081070No ratings yet
- The Optimized Effective Potential Method of Density Functional Theory: Applications To Atomic and Molecular SystemsDocument23 pagesThe Optimized Effective Potential Method of Density Functional Theory: Applications To Atomic and Molecular Systemstestonly261No ratings yet
- Space Curvilinear Motion FormulationDocument20 pagesSpace Curvilinear Motion FormulationSaqib Sher100% (1)
- Jackson Ch1 4 5 6 10 12 13Document11 pagesJackson Ch1 4 5 6 10 12 13Tom HoganNo ratings yet
- Skeletonization Using SSM of The Distance TransformDocument4 pagesSkeletonization Using SSM of The Distance TransformAryan SisodiaNo ratings yet
- A A A A - : 4.4 Potential EquationsDocument10 pagesA A A A - : 4.4 Potential EquationsteddyNo ratings yet
- To Solid State Physics: Prof. Igor Shvets Ivchvets@tcd - IeDocument39 pagesTo Solid State Physics: Prof. Igor Shvets Ivchvets@tcd - IeThiago Boimer CorreiaNo ratings yet
- Molecules Levels: Diatomic According TO Wave Mechanics. VibrationalDocument8 pagesMolecules Levels: Diatomic According TO Wave Mechanics. VibrationalSandipan SahaNo ratings yet
- 1986 Renormalization Group Analysis of Turbulence (5P)Document5 pages1986 Renormalization Group Analysis of Turbulence (5P)LeeSM JacobNo ratings yet
- Physics 2B For Materials and Structural EngineeringDocument52 pagesPhysics 2B For Materials and Structural EngineeringAnonymous 9uu04elNo ratings yet
- Chapter 4 Differintegration of Simple F - 1974 - Mathematics in Science and EngDocument8 pagesChapter 4 Differintegration of Simple F - 1974 - Mathematics in Science and EngFelipe Augusto Paes de GodoiNo ratings yet
- Lec 10Document4 pagesLec 10RKD CinemaNo ratings yet
- Semiconductor PhotonicsDocument30 pagesSemiconductor PhotonicswuasamomNo ratings yet
- PHYS500 Chapter01Document25 pagesPHYS500 Chapter01Levis CivitaNo ratings yet
- 100cia Tec 2020Document9 pages100cia Tec 2020fernandoNo ratings yet
- Gaussian Beamlet 2022 0Document24 pagesGaussian Beamlet 2022 0ROHIT ARORANo ratings yet
- Calculation of The Tightness of Flanged JointsDocument7 pagesCalculation of The Tightness of Flanged Jointspushpak_136No ratings yet
- Lukyanov 1995Document5 pagesLukyanov 1995Che ChoNo ratings yet
- Fick'S (First) Law of Binary Diffusiona: - P Vwai Cartesian Coordinates (XDocument3 pagesFick'S (First) Law of Binary Diffusiona: - P Vwai Cartesian Coordinates (XConsueloAndreaRiquelmeCarrascoNo ratings yet
- Aitken 1934Document5 pagesAitken 1934MariaUrtubiNo ratings yet
- Relativity of Pseudo-Spherical Concept and Hartree-Fock Concept For Condensed MatterDocument5 pagesRelativity of Pseudo-Spherical Concept and Hartree-Fock Concept For Condensed MatterIJRASETPublicationsNo ratings yet
- Lecture 6 CHEM 101 Fall 2023Document7 pagesLecture 6 CHEM 101 Fall 2023Pink PrintNo ratings yet
- Solution To HW ProblemsDocument4 pagesSolution To HW ProblemsAditya PrasadNo ratings yet
- Tutorial 10Document2 pagesTutorial 10pulkit mohataNo ratings yet
- IOQP-2020-21 aKAASH Vns RRR - (Answers & Solutions) - Part-2Document9 pagesIOQP-2020-21 aKAASH Vns RRR - (Answers & Solutions) - Part-2Vikash Kr YadavNo ratings yet
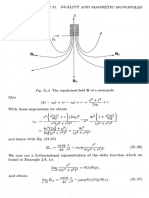
- Duality A ND Magnetic Monopoles: Fig. 21.4 The Regularised Field of MonopoleDocument1 pageDuality A ND Magnetic Monopoles: Fig. 21.4 The Regularised Field of MonopoleEddieNo ratings yet
- Duality A N D Magnetic MonopolesDocument1 pageDuality A N D Magnetic MonopolesEddieNo ratings yet
- The Delta: DistributionDocument1 pageThe Delta: DistributionEddieNo ratings yet
- Panorama B1 Deutsch Als Fremdsprache (Ulrike Würz)Document187 pagesPanorama B1 Deutsch Als Fremdsprache (Ulrike Würz)Neža Zupančič100% (2)
- POCSS ( ) S (Y S ( ) .: ConcludingDocument1 pagePOCSS ( ) S (Y S ( ) .: ConcludingEddieNo ratings yet
- Seem To Be Singular At: r3 (J ."TS"Document1 pageSeem To Be Singular At: r3 (J ."TS"EddieNo ratings yet
- M, Po (H + M) - H: Macroscopic MagnetostaticsDocument1 pageM, Po (H + M) - H: Macroscopic MagnetostaticsEddieNo ratings yet
- 411Document1 page411EddieNo ratings yet
- Chapter 21. Duality A N D Magnetic Monopoles: Regularisation The Monopole FieldDocument1 pageChapter 21. Duality A N D Magnetic Monopoles: Regularisation The Monopole FieldEddieNo ratings yet
- HW 3 CMDocument9 pagesHW 3 CMeddiejam1642No ratings yet
- AN THE Between Magnetic: Analytic Solution For Force TWO DipolesDocument14 pagesAN THE Between Magnetic: Analytic Solution For Force TWO DipolesEddieNo ratings yet
- Ch2 Coulomb's LawDocument44 pagesCh2 Coulomb's Lawmehdii.heidary136686% (7)
- Teoria de Grupos para Fisicos Rodolfo Alexander DiazDocument391 pagesTeoria de Grupos para Fisicos Rodolfo Alexander DiazGerman ChiappeNo ratings yet
- 14.9 Energy Transport in Wave Guides: D Z D L, Where DL Is An Element of The PathDocument1 page14.9 Energy Transport in Wave Guides: D Z D L, Where DL Is An Element of The PathEddieNo ratings yet
- 250 PDFDocument1 page250 PDFEddieNo ratings yet
- Solutions HW5Document4 pagesSolutions HW5Far Away Archipelago50% (4)
- Introduction EqDocument1 pageIntroduction EqEddieNo ratings yet
- Electromagnetic Waves in VacuumDocument1 pageElectromagnetic Waves in VacuumEddieNo ratings yet
- 14.5 Fundamental Equations The Coaxial Cable: GuidesDocument1 page14.5 Fundamental Equations The Coaxial Cable: GuidesEddieNo ratings yet
- The Comprehensive L TEX Symbol ListDocument338 pagesThe Comprehensive L TEX Symbol Listgabomath2010No ratings yet
- Chap 3Document17 pagesChap 3Yessenia Madrid100% (1)
- The Lihnard-Wiechert Potentials Moving Point Charge: J (R, Ro (T) )Document1 pageThe Lihnard-Wiechert Potentials Moving Point Charge: J (R, Ro (T) )EddieNo ratings yet
- Teoria de Grupos para Fisicos Rodolfo Alexander DiazDocument391 pagesTeoria de Grupos para Fisicos Rodolfo Alexander DiazGerman ChiappeNo ratings yet
- Magne Tostatics: B (R) - K'V (IR)Document1 pageMagne Tostatics: B (R) - K'V (IR)EddieNo ratings yet
- 33 PDFDocument1 page33 PDFEddieNo ratings yet
- (E - D + H - B) V - (E X H) + J - e .: R, L I RDocument1 page(E - D + H - B) V - (E X H) + J - e .: R, L I REddieNo ratings yet
- Themaxwell Equations: LoopDocument1 pageThemaxwell Equations: LoopEddieNo ratings yet
- On The Choice of Units: But atDocument1 pageOn The Choice of Units: But atEddieNo ratings yet
- Solutions Manual To Irodov General Problems in Physics - Mechanics - Problems From 76 To 100Document30 pagesSolutions Manual To Irodov General Problems in Physics - Mechanics - Problems From 76 To 100EddieNo ratings yet