Professional Documents
Culture Documents
المتفوق 01 by DEBILI Samir Lycee Malek Bennabi TEBESSA
Uploaded by
Amdjed Bahaa0 ratings0% found this document useful (0 votes)
52 views1 pageيييثص
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentيييثص
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
52 views1 pageالمتفوق 01 by DEBILI Samir Lycee Malek Bennabi TEBESSA
Uploaded by
Amdjed Bahaaيييثص
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
: :
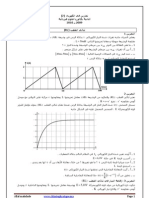
-1 0,10 mole CH3CH2COOH 500 mL .I L B r
S0 E=6V k . R=110 t= 0
. C ) i(t . 01
PH 2.8 -1 - ) UB(t
. ). UR(t
K PKa acide/base -
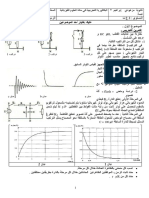
-2 0,20 mole 0,20 mole -1- butan-1- i
ol C4H10O . di(t)/dt+ /L i(t)= E/L
. r .R
. -
) i(t)= I0(1- / :
-3 = t ) I0=E/(R+r ) = /( +
4.9g . -2 I0
. L . r
. -3
( L R )E 02
-/1: ) ( 238U
-
) ( 20682Pb ) ( ).(- . .
: .II
238 206
92U x+y + 82Pb E R0=20 R1
) ( t =0 ) NU(0 ) (t ) NU(t K c=0.110F
L . r=5
-/ ) (x ) ( y -1 ( )1
-/ . ( )2 . t=0
-/ N=N0/16 t=4 t1/2 - .
-/ ) ( t -
) NPb(t) =NU(0) (1 e-t ) i(t .
-/2 -2 UR0 - T L
. -/ - )q(t
-/ m = 1 g - ) Uc(t
-/ 30
- t=9T/8 .
p = 25 x106w m(U) = 238.0003u :
)Ub(t
m(Pb)=205.9295u m(He)=4.0015u m(e)=0.00054uA=6.023x1023 mol-1
1 MeV=1.6x10-13j 1u=931.5Mev
You might also like
- Révision BAC 2018Document172 pagesRévision BAC 2018ZineChini100% (5)
- 8Document2 pages8e.maskarNo ratings yet
- الفرض المنزلي رقم 1 الدورة الثانية ثانية علوم الحياة والأرض 1 و 2Document2 pagesالفرض المنزلي رقم 1 الدورة الثانية ثانية علوم الحياة والأرض 1 و 2الغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- التحولات ح,ق و الكهرباءDocument1 pageالتحولات ح,ق و الكهرباءYassine0% (1)
- lateur1الأختبار الثاني 2008-2009 الجزائرDocument2 pageslateur1الأختبار الثاني 2008-2009 الجزائرassembleur7777100% (1)
- 3 3 (Aq) 3 + (Aq) - (Aq) 3 3 (Aq) 2 (L) - (Aq) 3 + (Aq) - 1Document10 pages3 3 (Aq) 3 + (Aq) - (Aq) 3 3 (Aq) 2 (L) - (Aq) 3 + (Aq) - 1chaimaNo ratings yet
- JDGDHDocument1 pageJDGDHYassineNo ratings yet
- سلسلة تمارين في العلوم الفيزيائية للسنة الثالثة ثانويDocument38 pagesسلسلة تمارين في العلوم الفيزيائية للسنة الثالثة ثانويالغزيزال الحسن EL GHZIZAL Hassane100% (5)
- BBL AbedDocument4 pagesBBL Abedعبد العزيز مروىNo ratings yet
- حالة توازن و التحولات النوويةDocument2 pagesحالة توازن و التحولات النوويةRachid SadNo ratings yet
- حالة توازن و التحولات النوويةDocument2 pagesحالة توازن و التحولات النوويةRachid SadNo ratings yet
- Contr 1ér S 3-2 2bacDocument2 pagesContr 1ér S 3-2 2bacyoucard50% (2)
- Exo TerminalDocument11 pagesExo TerminalshadowNo ratings yet
- Cmpo1 WadiDocument4 pagesCmpo1 Wadiعبد العزيز مروىNo ratings yet
- فرض رقم 2 السنة الثانية ع - فيزيائيةDocument2 pagesفرض رقم 2 السنة الثانية ع - فيزيائيةالغزيزال الحسن EL GHZIZAL Hassane100% (5)
- 1799693 فرض رقم 2 السنة الثانية ع فيزيائيةDocument2 pages1799693 فرض رقم 2 السنة الثانية ع فيزيائيةessadikine anassNo ratings yet
- 5 1 08Document1 page5 1 08smjamal100% (1)
- الدارة Rlc القسرية التضمين و الكيمياءDocument2 pagesالدارة Rlc القسرية التضمين و الكيمياءعبدالرزاق اغزيلNo ratings yet
- TD UdDocument2 pagesTD UdboustakatbNo ratings yet
- 3as Phys Be 2017 2Document8 pages3as Phys Be 2017 2Mohamed taha EL M'HAMDINo ratings yet
- 2s CompoDocument2 pages2s Compoamine milanoNo ratings yet
- Abb 2Document1 pageAbb 2Amel HbNo ratings yet
- Physics 3se18 1trim4Document3 pagesPhysics 3se18 1trim4Mohamed LoghbiNo ratings yet
- فرض منزلي رقم 1 الدورة الثانيةDocument3 pagesفرض منزلي رقم 1 الدورة الثانيةالغزيزال الحسن EL GHZIZAL Hassane100% (6)
- التوزنات الكيميائية و التحولات النووية 10Document1 pageالتوزنات الكيميائية و التحولات النووية 10YassineNo ratings yet
- Examen2 smb1Document2 pagesExamen2 smb1Amine AlaoUii AlaouiNo ratings yet
- الفرض1للدورة 2Document3 pagesالفرض1للدورة 2AliNo ratings yet
- Compo2 IARDocument6 pagesCompo2 IARعبد العزيز مروىNo ratings yet
- Devoir3 SVTDocument4 pagesDevoir3 SVTSiMo ElbNo ratings yet
- ثالثة تر التبسيDocument2 pagesثالثة تر التبسيWissam GouasmiaNo ratings yet
- Bac Physics 2016 Se 2Document8 pagesBac Physics 2016 Se 2abdelhak AouadiNo ratings yet
- تمارينات المتابعة الزمنية لتحول كيميائي للأستاذ التيجاني دهام PDFDocument6 pagesتمارينات المتابعة الزمنية لتحول كيميائي للأستاذ التيجاني دهام PDFAmine ChettafNo ratings yet
- Contr 1ér S 3-1 2bacDocument2 pagesContr 1ér S 3-1 2bacyoucard100% (2)
- Physics 2mtm20 1trim2Document2 pagesPhysics 2mtm20 1trim2Amel HbNo ratings yet
- Physics 2se20 1trim1Document2 pagesPhysics 2se20 1trim1Zouaoua SlimaneNo ratings yet
- Physics 2mtm20 1trim2Document2 pagesPhysics 2mtm20 1trim2Amel HbNo ratings yet
- امتحان تجريبي بكالوريا التعليم الثانوي دورة ماي 2014Document10 pagesامتحان تجريبي بكالوريا التعليم الثانوي دورة ماي 2014الغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- فرض محروس رقم 3 الدورة الأولى ع ف 2012 2013 2 PDFDocument2 pagesفرض محروس رقم 3 الدورة الأولى ع ف 2012 2013 2 PDFKhairLatamnaNo ratings yet
- الموضوعDocument8 pagesالموضوعallalmayar2022No ratings yet
- الدارة rlc القسرية التضمين و الكيمياءDocument3 pagesالدارة rlc القسرية التضمين و الكيمياءMofid PCNo ratings yet
- Execices RL SM Classe 10 - 11 AllalDocument5 pagesExecices RL SM Classe 10 - 11 AllalkeomatNo ratings yet
- العلوم الفيزيائيةDocument8 pagesالعلوم الفيزيائيةmouradNo ratings yet
- Execices RLC SM Classe 10 - 11 AllalDocument9 pagesExecices RLC SM Classe 10 - 11 AllalkeomatNo ratings yet
- Execices RLC SM Classe 10 - 11 AllalDocument9 pagesExecices RLC SM Classe 10 - 11 AllalZakaria ElhammoumiNo ratings yet
- Examin 000Document2 pagesExamin 000dam 12No ratings yet
- التحولات حمض قاعدة و الكهرباء 5Document1 pageالتحولات حمض قاعدة و الكهرباء 5HarounSamihNo ratings yet
- اختبار الفصل 2 مرفق بالحلDocument8 pagesاختبار الفصل 2 مرفق بالحلYoucef JousephNo ratings yet
- RCتنائي القطبDocument1 pageRCتنائي القطبMohamed KhoujmaniNo ratings yet
- Exam 2021Document2 pagesExam 2021سمير دبيليNo ratings yet
- Compo3Prem SoumiaDocument3 pagesCompo3Prem SoumiaManou100% (1)
- 2 ExaminDocument3 pages2 Examindam 12No ratings yet
- فرض محروس 2 ع رDocument1 pageفرض محروس 2 ع رMajed GharibNo ratings yet
- Composition de Physique 3AS - Sujet 12Document2 pagesComposition de Physique 3AS - Sujet 12Hasan RajawiNo ratings yet
- Compo213 BrahimDocument4 pagesCompo213 BrahimAymen DjellalNo ratings yet
- إمتحان المنافسة العلمية في مادة العلوم الفيزيائية 2010Document9 pagesإمتحان المنافسة العلمية في مادة العلوم الفيزيائية 2010Is LamNo ratings yet
- تمرين حول حجم غاز,Document2 pagesتمرين حول حجم غاز,Achref SaidNo ratings yet
- Dev3 TiziDocument1 pageDev3 TiziAmdjed BahaaNo ratings yet
- Bbl15M RahemDocument4 pagesBbl15M RahemAmdjed BahaaNo ratings yet
- 3Document27 pages3LaziriAbdelhalim60% (5)
- Bac 2017 SDocument8 pagesBac 2017 SAmdjed BahaaNo ratings yet
- 03Document16 pages03Amdjed BahaaNo ratings yet
- RC17Document15 pagesRC17Amdjed BahaaNo ratings yet
- Dev 2 2emme Asse2 Lycee Fatma Zohra Prof DEBILI SDocument2 pagesDev 2 2emme Asse2 Lycee Fatma Zohra Prof DEBILI SAmdjed BahaaNo ratings yet
- التمرين التجريبيDocument4 pagesالتمرين التجريبيAmdjed BahaaNo ratings yet
- Dev 2 2emme Asse2 Lycee Fatma Zohra Prof DEBILI SDocument2 pagesDev 2 2emme Asse2 Lycee Fatma Zohra Prof DEBILI SAmdjed BahaaNo ratings yet
- 01Document2 pages01Amdjed BahaaNo ratings yet
- المتفوق 2Document1 pageالمتفوق 2Amdjed BahaaNo ratings yet
- Arabic1am I3rab PDFDocument15 pagesArabic1am I3rab PDFAmdjed BahaaNo ratings yet
- Guid 2ap Educ Esl Civ 15-06-2016 Said PDFDocument64 pagesGuid 2ap Educ Esl Civ 15-06-2016 Said PDFAmdjed BahaaNo ratings yet
- أحافظ على صحّتي دفتر الأنشطةDocument1 pageأحافظ على صحّتي دفتر الأنشطةAmdjed BahaaNo ratings yet
- 9bqer - 1Document4 pages9bqer - 1Amdjed BahaaNo ratings yet
- فهرس س2 كتاب التلميذ PDFDocument4 pagesفهرس س2 كتاب التلميذ PDFAmdjed BahaaNo ratings yet
- المتابعة الزمنية لتحول كيميائيDocument3 pagesالمتابعة الزمنية لتحول كيميائيAmdjed BahaaNo ratings yet
- مقتطفات من دليل الأستاذ س2 رياضياات تربية علميةDocument20 pagesمقتطفات من دليل الأستاذ س2 رياضياات تربية علميةAmdjed BahaaNo ratings yet
- الدليل مظاهر التنفسDocument1 pageالدليل مظاهر التنفسAmdjed BahaaNo ratings yet
- كتاب التلميذ تفكيك جمعي للأعداد إلى 69Document1 pageكتاب التلميذ تفكيك جمعي للأعداد إلى 69Amdjed BahaaNo ratings yet
- 9bu0k-Unite 02 SerierDocument4 pages9bu0k-Unite 02 SerierAmdjed BahaaNo ratings yet
- Wahy El-Qolam Vol. 1Document346 pagesWahy El-Qolam Vol. 1Muhammad NurthariqNo ratings yet
- ActivFrotSec AmiraDocument1 pageActivFrotSec AmiraAmdjed BahaaNo ratings yet
- Hisgeo 1am2fDocument1 pageHisgeo 1am2fAmdjed BahaaNo ratings yet
- Hisgeo 1am2fDocument1 pageHisgeo 1am2fAmdjed BahaaNo ratings yet
- أكمل الفراغDocument1 pageأكمل الفراغAmdjed BahaaNo ratings yet
- 1asa BelkheirDocument2 pages1asa BelkheirzemoussaNo ratings yet
- الفرض الرابع السنة الثانيةDocument1 pageالفرض الرابع السنة الثانيةAmdjed BahaaNo ratings yet