Professional Documents
Culture Documents
FileAssignment Tugas Kinetika Kimia PDF
FileAssignment Tugas Kinetika Kimia PDF
Uploaded by
Irvan Fanani0 ratings0% found this document useful (0 votes)
20 views3 pagesThe document discusses two chemical reactions:
1) The decomposition of nitrogen dioxide gas over 150 seconds. The initial concentration was 0.0100 mol/L and after 150 seconds was 0.0055 mol/L.
2) The reaction of S2O82- and I- ions to form sulfate and triiodide ions. Three experiments were conducted with varying concentrations and initial rates were measured.
Original Description:
Original Title
FileAssignment_Tugas_Kinetika_Kimia.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses two chemical reactions:
1) The decomposition of nitrogen dioxide gas over 150 seconds. The initial concentration was 0.0100 mol/L and after 150 seconds was 0.0055 mol/L.
2) The reaction of S2O82- and I- ions to form sulfate and triiodide ions. Three experiments were conducted with varying concentrations and initial rates were measured.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
20 views3 pagesFileAssignment Tugas Kinetika Kimia PDF
FileAssignment Tugas Kinetika Kimia PDF
Uploaded by
Irvan FananiThe document discusses two chemical reactions:
1) The decomposition of nitrogen dioxide gas over 150 seconds. The initial concentration was 0.0100 mol/L and after 150 seconds was 0.0055 mol/L.
2) The reaction of S2O82- and I- ions to form sulfate and triiodide ions. Three experiments were conducted with varying concentrations and initial rates were measured.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 3
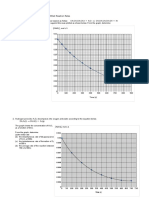
The Decomposition of Nitrogen Dioxide
Average Rate
• Consider the following reaction at 300oC:
2 NO2(g) → 2 NO(g) + O2(g)
• The initial concentration of NO2 is 0.0100 mol/L and
its concentration after 150 s is 0.0055 mol/L. What
are the average rates of this reaction during the first
150 s and during the second 150 s?
Determination of Rate Law using Initial Rate
Reaction: S2O82-(aq) + 3I- (aq) → 2SO42- (aq) + I3- (aq)
• The following data were obtain.
• ⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯
• Expt. [S2O82-] [I-] Initial Rate,
• # (mol/L) (mol/L) (mol/L.s)
• ⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯
• 1 0.036 0.060 1.5 x 10-5
• 2 0.072 0.060 2.9 x 10-5
• 3 0.036 0.120 2.9 x 10-5
• ⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯
You might also like
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- 5.1.1 Exercise 1 - CONCENTRATION-TIME GRAPHSDocument2 pages5.1.1 Exercise 1 - CONCENTRATION-TIME GRAPHSIqbal Hossain ZahidNo ratings yet
- AP Chapter 14 JeopardyDocument33 pagesAP Chapter 14 JeopardyLama DebanyNo ratings yet
- Chemical Kinetics ExercisesDocument2 pagesChemical Kinetics ExercisesBanana CrazyNo ratings yet
- Chapter 12 Chemical KineticsDocument90 pagesChapter 12 Chemical KineticsGurudutt SharmaNo ratings yet
- By Einar Helland Berger (Own Work) (CC BY SA 2.5 ), Via Wikimedia CommonsDocument49 pagesBy Einar Helland Berger (Own Work) (CC BY SA 2.5 ), Via Wikimedia CommonsHoàng Anh DbbyNo ratings yet
- Assignment 1-Solution and Solubility: D. 0.150 Molal MGCL (Aq)Document3 pagesAssignment 1-Solution and Solubility: D. 0.150 Molal MGCL (Aq)utpNo ratings yet
- CHE1010 Tutorial Sheet 7Document3 pagesCHE1010 Tutorial Sheet 7Chimuka Onson MapikiNo ratings yet
- CRE Assignment 1Document3 pagesCRE Assignment 1AkashTripathiNo ratings yet
- bài tập rateDocument2 pagesbài tập rateMys Genie100% (1)
- Chemcal Kinetics (Tutorial Questions)Document3 pagesChemcal Kinetics (Tutorial Questions)renNo ratings yet
- Homework Gases Mel Arthor QueditDocument2 pagesHomework Gases Mel Arthor QueditMel Arthor QueditNo ratings yet
- AP Chemistry - Iodine Clock Reaction Lab ReportDocument4 pagesAP Chemistry - Iodine Clock Reaction Lab ReportJustin MorrowNo ratings yet
- Group No. (4) Exp. No. (11) Date:-17/12/2008 Exp Name: - Chemical KineticsDocument4 pagesGroup No. (4) Exp. No. (11) Date:-17/12/2008 Exp Name: - Chemical Kineticsشركة العاصمة لخدمات التنظيفNo ratings yet
- CHEM 154 PROBLEM SET 1 - Chemical Kinetics - September 2018Document1 pageCHEM 154 PROBLEM SET 1 - Chemical Kinetics - September 2018AlyssaRamosNo ratings yet
- Problem Set 2Document2 pagesProblem Set 2Adrian PazNo ratings yet
- CalculationsDocument9 pagesCalculationsCharlotte SamilinNo ratings yet
- ChemistryDocument3 pagesChemistrySomeday -No ratings yet
- Sodium Hypochlorite (: The ChemistryDocument3 pagesSodium Hypochlorite (: The ChemistryTrishia Justine BattungNo ratings yet
- Kinetics Practice ProblemsDocument4 pagesKinetics Practice ProblemsLuke MeredithNo ratings yet
- Chemical Kinetics: Chapter 14.1-2Document22 pagesChemical Kinetics: Chapter 14.1-2Sagar GurowNo ratings yet
- 110 WS Gas Stoichiometry KeyDocument2 pages110 WS Gas Stoichiometry KeyDestiny Marie NavarroNo ratings yet
- 110 WS Gas Stoichiometry KeyDocument2 pages110 WS Gas Stoichiometry Keyエルミタ ジョイ ファティマ100% (1)
- G-12 Chemistry Exam (Skill and Memory Testing)Document1 pageG-12 Chemistry Exam (Skill and Memory Testing)mohammed hassen mohammedNo ratings yet
- Facultad de Ingeniería Y Ciencias Geológicas Departamento de Ingeniería Metalúrgica y MinasDocument6 pagesFacultad de Ingeniería Y Ciencias Geológicas Departamento de Ingeniería Metalúrgica y MinasCristian Serrano ArayaNo ratings yet
- Midterm Exam Location Odette Building, Room 104 Feb. 3rd From 1 - 2:20 P.M. March 10th From 1 - 2:20 P.MDocument6 pagesMidterm Exam Location Odette Building, Room 104 Feb. 3rd From 1 - 2:20 P.M. March 10th From 1 - 2:20 P.Md_94No ratings yet
- CHM 111 Chemical Kinetics Prof. SoriyanDocument34 pagesCHM 111 Chemical Kinetics Prof. SoriyanOluwaferanmi OgunleyeNo ratings yet
- 2011-2012 Prequiz For Kinetics - Problems and SolutionsDocument7 pages2011-2012 Prequiz For Kinetics - Problems and SolutionsJomari GaliasNo ratings yet
- Ce311a Lec 15 Ozone FormationDocument14 pagesCe311a Lec 15 Ozone FormationGaurav SinghNo ratings yet
- Reaction MechanismDocument37 pagesReaction MechanismNurshuhada NordinNo ratings yet
- Kinetics PDFDocument6 pagesKinetics PDFRichard QiuNo ratings yet
- Ejercicios Resueltos InglesDocument4 pagesEjercicios Resueltos InglesAmparo OssaNo ratings yet
- Assignment 3 Chemical KineticsDocument1 pageAssignment 3 Chemical Kineticsvegamaharajfaith02No ratings yet
- 163Ch11 13Document7 pages163Ch11 13Aaron BautistaNo ratings yet
- Chemical KineticsDocument22 pagesChemical KineticsEleanorNo ratings yet
- Chem 155 Tutorial Questions 4Document3 pagesChem 155 Tutorial Questions 4Abede Saviour DelaliNo ratings yet
- CHM270 - Tutorial 3 (Chemical Kinetics)Document7 pagesCHM270 - Tutorial 3 (Chemical Kinetics)Azrie HizadNo ratings yet
- Chem 73 3rd Exam 2010 Answers-1Document2 pagesChem 73 3rd Exam 2010 Answers-1Gabriel Billones Jr.No ratings yet
- Tutorial 1Document1 pageTutorial 1Aisyah ShaariNo ratings yet
- CM1502 Tutorial 6: P, M 2 - 1 - 1 2 - 1 - 1 2 - 1 - 1 Fus - 1 Vap - 1Document2 pagesCM1502 Tutorial 6: P, M 2 - 1 - 1 2 - 1 - 1 2 - 1 - 1 Fus - 1 Vap - 1Jim HippieNo ratings yet
- Ex - Chapter 4,5 - Kinetics and Catalysis-2023.2-NewDocument1 pageEx - Chapter 4,5 - Kinetics and Catalysis-2023.2-NewKhánh DuyNo ratings yet
- 5bfd1a25-a358-45a3-b994-02540a001a19Document2 pages5bfd1a25-a358-45a3-b994-02540a001a19Student KeekNo ratings yet
- Determining The Rate Law From Experimental DataDocument45 pagesDetermining The Rate Law From Experimental Datasospeter barasaNo ratings yet
- Chemical Kinetics: Joshua Micole Felizarta, LPTDocument25 pagesChemical Kinetics: Joshua Micole Felizarta, LPTArvin SayotoNo ratings yet
- Absorption of CO2 - 1Document9 pagesAbsorption of CO2 - 1Sanskriti GhisingNo ratings yet
- Tutorial 1bDocument2 pagesTutorial 1bKamilia AfiqahNo ratings yet
- Sk0014 Physical & Iinorganic Chemistry Tutorial 4: Reaction KineticsDocument2 pagesSk0014 Physical & Iinorganic Chemistry Tutorial 4: Reaction KineticsNeil8353 GgNo ratings yet
- Tutorial 2 ThermochemistryDocument1 pageTutorial 2 ThermochemistrykrishanuNo ratings yet
- Drills For An A CHM096Document20 pagesDrills For An A CHM096Aiman FitryNo ratings yet
- Tutorial 2Document2 pagesTutorial 2EreenNo ratings yet
- 142A Practiceexam3 W20KEYDocument3 pages142A Practiceexam3 W20KEYAlana Yudha-WrightNo ratings yet
- Chpt13 SEDocument4 pagesChpt13 SELillaNo ratings yet
- Chemistry Worksheet 2Document3 pagesChemistry Worksheet 2LemontNo ratings yet
- St00502 Basic Chemistry Assignment 2 Answer All of The QuestionsDocument2 pagesSt00502 Basic Chemistry Assignment 2 Answer All of The QuestionsOri LukeNo ratings yet
- 5 Solution Stoichiometry (S)Document11 pages5 Solution Stoichiometry (S)Mr TanNo ratings yet
- RedoxDocument30 pagesRedoxMelanie perez cortezNo ratings yet
- Determining Activation EnergyDocument5 pagesDetermining Activation Energyxyzxd0% (1)
- Answers Rates of Reactions Practice QuestionsDocument4 pagesAnswers Rates of Reactions Practice Questionsعوض أمحمدNo ratings yet
- Quantitative Determination of Dissolved Oxygen Content by Winkler Redox TitrationDocument5 pagesQuantitative Determination of Dissolved Oxygen Content by Winkler Redox TitrationJemimahNo ratings yet