Professional Documents
Culture Documents
Title of Lecture: AY 2018-2019 1 Shifting Exam Instructor's Name (Name, MD, Other-Post-Nominals) Mm/Dd/Yyyy
Uploaded by
Marielle MalabananOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Title of Lecture: AY 2018-2019 1 Shifting Exam Instructor's Name (Name, MD, Other-Post-Nominals) Mm/Dd/Yyyy
Uploaded by
Marielle MalabananCopyright:
Available Formats
Pathology Title of Lecture
AY 2018-2019 Instructor’s Name (Name, MD, other-post-nominals)
1st Shifting Exam MM/DD/YYYY
OUTLINE
Note: For long outlines, use two columns to save space for main
content. For short outlines, just merge the two columns.
LEARNING OBJECTIVES
Note: If no learning objectives were given during the lecture, either
use the ones in the handout given or delete this portion altogether
VI. ADRENAL CORTEX
Has three zones: zona glomerulosa (mineralocorticoids), zona
fasciculata (glucocorticoids), zona reticularis (sex steroids).
Figure X. Endogenous causes of Cushing syndrome
Morphology:
Depending on the cause of the hypercortisolism, the
adrenals show one of the following abnormalities: (1) cortical
Figure X. Adrenal Glands atrophy, (2) diffuse hyperplasia, (3) macronodular or
micronodular hyperplasia, and (4) an adenoma or carcinoma.
A. ADRENOCORTICAL HYPERFUNCTION Hyperplasia is always bilateral and adenoma is unilateral
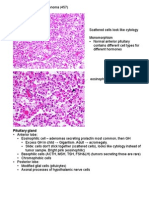
Crooke’s hyaline change
May arise from 3 different conditions: Seen in Pituitary Hypercorticolism; most common
Cushing syndrome: excessive cortisol alteration in pituitary gland
Hyperaldosteronism: excessive aldosterone ACTH producing cells’ basophilic cytoplasm in the anterior
Virilizing syndromes or adrenogenital: excess of pituitary becomes homogeneous and pale due to the
androgens accumulation of intermediate keratin filaments in the
cytoplasm.
1. HYPERCORTISOLISM
“Cushing’s Syndrome”
Causes:
Exogenous
The most common cause is exogenous administration of
exogenous glucocorticoids (“iatrogenic” Cushing
syndrome).
Ex. Treatment of allergies or asthma
Endogenous
1. ACTH dependent
70% of cases of endogenous hypercortisolism Figure X. Crooke’s hyaline change
Pituitary neoplasms are the most common underlying Abnormalities of adrenal gland:
causes (70% of all endogenous cases) Cortical atrophy
Cushing Disease, Corticotropin syndrome Iatrogenic Cushing syndrome
2. ACTH independent Suppression of endogenous ACTH lack of
Uncommon stimulation of the zona fasciculata and reticularis
Ex. Macronodular hyperplasia where cortisol cortical atrophy
production is regulated by non- ACTH circulating Diffuse hyperplasia
hormones, due to ectopic overexpression of their ACTH-dependent Cushing syndrome
corresponding receptors in the adrenocortical cells Both glands are enlarged
S# T# Group # : Surname, Surname, Surname 1 of 5
Hyperplastic cortex shows expanded lipid-poor zona
reticularis with compact eosinophilic cells surrounded
by outer zone of vacuolated “lipid-rich” cells
Macronodular hyperplasia
The adrenals are almost entrirely replaced by
prominent nodules of varying sizes, which contain an
admixture of lipid-poor and lipid-rich cells
Figure X. Adrenocortical carcinoma. Shows marked
Figure X. Macronodular hyperplasia. The nodules are filled with degree of anaplasia and pleomorphism in the nucleus.
lipid rich areas because the precursors of the hormones are
cholesterol, thus, they need more lipid. Clinical Manifesations:
Central pattern of adipose tissue deposition which becomes
Micronodular hyperplasia apparent in the form of truncal obesity, moon facies, and
Darkly pigmented (pigments composed of brown to buffalo hump. (most common)
black- lipofuscin) micronodules, with atrophic intervening Decrease in muscle mass and proximal limb weakness
areas. Hyperglycemia, glucosuria, and polydipsia (secondary
Not much of a change when it comes to size diabetes)
Clinical Course
Develops slowly; early stages: hypertension and weight gain
Pattern of adipose deposition is distinct: truncal obesity,
moon facies, and accumulation of fat in the posterior neck
and back (buffalo hump)
Selective atrophy of fast-twitch (type 2) myofibers → ↓muscle
mass and proximal limb weakness
Figure X. Micronodular hyperplasia. (Right) you can observe the Glucocorticoids induce gluconeogenesis → inhibit uptake of
accumulation of lipofuscin on the far left. glucose → hyperglycemia, glucosuria and polydipsia
(secondary diabetes)
Primary Adrenocortical neoplasms Catabolic effects: loss of collagen, resorption of bones
Malignant/ benign Skin: thin, fragile, easily bruised, poor wound healing,
More common in women in 30s – 50s cutaneous striae in abdominal area
Adrenocortical adenomas: yellow tumors surrounded by Bone resorption → osteoporosis, backache, ↑susceptibility
capsule; cells are similar to normal zona fasciculata to fracture
To differentiate it from carcinoma, one of the basis is Mental disturbances: mood swings, depression, and frank
the SIZE, most of the time 30 grams is an adenoma psychosis
and more than 300 grams it becomes a carcinoma. Hirsutism & menstrual abnormalities in women
Resemble the cells found in zona fasciculate
Contain numerous vacuolated cells because due to the Laboratory Diagnosis:
lipids present Inc. 24 hour urine free cortisol concentration
Carcinoma: unencapsulated masses that have all of the Loss of normal diurnal pattern of cortisol secretion
anaplastic characteristics of cancer Normally the cortisol is expected to be increasing as the
Tend to be larger than the adenomas; poorly day goes on and goes down at the end of the day but in
demarcated lesions containing areas of necrosis, Cushing’s syndrome it is persistently elevated.
hemorrhage, and cystic change. Determining the cause of Cushing’s syndrome depends on
ACTH secretion and measurement of urinary steroids after
Dexamethasone administration (Dexamethasone Test)
By performing Dexamethasone suppression test you can
localize where the problem is
Dexamethose Suppression Test is used to suppress the release of
ACTH from your anterior pituitary. With this, the only syndrome
here wherein there’s an increase release of ACTH is the Cushing’s
Syndrome. The rest is due to ectopic releases or adrenal tumors
that are outside the brain so they are not affected. Thus, this is a
test to detect Cushing’s Syndrome.
Figure X. Gross picture of adrenal cortical adenoma. Is is
distinguished from nodular hyperplasia by its solitary, well- General Pattern of Tests:
1. Pituitary Hypercorticolism
circumscibed nature. Basis is the size!
Low dose dexamethasone: ↑ACTH and can’t be suppressed,
no reduction in urinary excretion of 17-hydroxycorticosteroids
Patho Title of Lecture 2 of 5
High dose of dexamethasone: ↓ACTH secretion,
suppression of urinary steroid secretion
2. Ectopic ACTH
↑ACTH, but its secretion is completely insensitive to low or
high doses of exogenous dexamethasone
Seen in some of the paraneoplastic syndromes (certain form
of cancers)
Not related to adrenal or pituitary but rather to the tumor that
is producing the ACTH
Insensitive to low or high doses of Dexamethasone
3. Adrenal tumor
Low or high doses of Dexamethasone cannot suppress Figure X. Gross: Conn’s Syndrome. Almost always
cortisol excretions; Insensitive to high and low doses of solitary, small, well-circumscribed lesions that are bright yellow on
dexamethasone cut section composed of lipid-laden cortical cells.
In general, ACTH levels are low due to feedback inhibition
Aldosterone-producing adenoma
Table X. Pattern of results for different diseases with the Almost always solitary, small (<2cm), well circumscribed lesions
Dexamethasone suppression test
More often found on the left than on right adrenal gland
Disease Low-dose High-dose ACTH
Bright yellow in cut section, composed of lipid laden cortical
Dexamethasone Dexamethasone
cells
Cushing’s Non-responsive Responsive High
Presence of eosinophilic, laminated cytoplasmic inclusions
Disease
known as spironolactone bodies upon treatment with
Ectopic ACTH Non-responsive Non-responsive High
spironolactone
secretion
Does not usually express ACTH secretion
Adrenal Non-responsive Non-responsive Low
Tumor
Glucocorticoid-remediable hyperaldosteronism
Uncommon cause of primary familial hyperaldosteronism
2. HYPERALDOSTERONISM
Involves chromosome 8, CYP11B2 (encoding aldosterone
synthase) under control of ACTH responsive CYP11B1 gene
Generic term for a group of closely related conditions promoter
characterized by chronic excess aldosterone secretion. Unusual circumstance – aldosterone production under
It may be primary or secondary (extra-adrenal cause). control of ACTH – thus, dexamethasone suppressible
Endpoint: HYPERTENSION
PRIMARY HYPERALDOSTERONISM
Primary – autonomous overproduction of aldosterone, with
resultant suppression of the RAAS and ↓ renin activity
High plasma aldosterone, LOW plasma renin
Blood pressure elevation is the most common manifestation of
primary hyperaldosteronism secondary to increased
aldosterone production.
Caused by 3 mechanisms:
Bilateral Idiopathic hyperaldosteronism
Older > younger population characterized by bilateral
nodular hyperplasia
Most common underlying cause, accounting for 60% of
cases
Less severe hypertension than with adrenal neoplasms
Unclear pathogenesis but recently KCNJ5 mutation, a
potassium channel
Diffuse, focal hyperplasia of cells resembling those of
normal zone glomerulosa
Wedge-shaped, extending from the periphery towards the
center of the gland
Adrenocortical neoplasm
May be an aldosterone adenoma or rarely adrenocortical
carcinoma
Adenoma – 2nd most common cause
Glucocorticoid suppressible adenoma in the pituitary
causing excessive ACTH release
35% by solitary aldosterone-secreting adenoma known
as Conn Syndrome
Middle life, F > M (2:1); KCNJ5 mutation also present Figure X. Mechanisms of primary hyperaldosteronism
SECONDARY HYPERALDOSTERONISM
Secondary – aldosterone release in response to the activation
of RAAS due to:
Increased levels of renin
Patho Title of Lecture 3 of 5
Decreased renal perfusion (arteriolar nephrosclerosis, renal If screening test is positive, confirmatory aldosterone
artery stenosis) suppression test must be performed
Arterial hypovolemia and edema (CHF, cirrhosis, nephrotic
syndrome); dehydration, hemorrhage, 3. ANDROGENITAL SYNDROMES
Pregnancy (estrogen-induced increase in plasma renin There are 2 important enzymes: 21-alpha hydroxylase and 17-
substrate) – may increase levels of renin hydroxyprogesterone. Blockage of the 21-alpha will result in
Related to any condition that could increase renin in the salt-wasting because there is no aldosterone. When the 2
blood as in cases of decreased renal perfusion (Severe renal enzymes are blocked, there will be an increase in sex hormone
artery stenosis) production leading to a virilised male or female.
High plasma aldosterone AND high plasma renin Female will grow facial hairs, hair on extremities and menstrual
problem. In men, alopecia, sexual dysfunction.
Morphology: 2 kinds: adrenocortical neoplasia or congenital adrenal
Aldosterone-producing adenoma – Spironolactone bodies hyperplasia
Lipid-laden cortical cells closely resembling the fasciculata The adrenal cortex secretes two compounds –
cells than the glomerulosa (source of aldosterone) dihydroepiandosterone and androstenedione which require
Cells tends to be uniform in size and shape with some conversion to testosterone in peripheral tissues for androgenic
nuclear and cellular pleomorphism effects. Adrenal androgen formation is regulated by ACTH; thus,
excessive secretion can present as an isolated syndrome or in
combination with features of Cushing’s disease.
Adrenocortical neoplasia associated with virilization are more
likely to be androgen-secreting adrenal carcinomas than
adenomas
a. CONGENITAL ADRENAL HYPERPLASIA
Autosomal recessive inherited metabolic errors leading to a lack
of enzyme particularly cortisol synthesis
Leads to a compensatory increases in ACTH secretion due to
absence of feedback inhibition
21-hydroxylase deficiency – salt losing syndrome; simple
virilising adrenogenital syndrome without salt wasting; involves
mutation of the CYP21A2 gene
Non-classic or late onset adrenal virilism – leading to increased
activity of 17-hydroxylase
Morphology:
The adrenals are hyperplastic bilaterally sometimes
expanding 10-15 times their normal weights
Figure X. Spirolactone bodies – eosinophilic, laminated
cytoplasmic inclusion in the cortex. Adrenal cortex is thickened and nodular, widened cortex on
cut section is brown due to lipid deposition
Clinical course: Proliferating cells are compact, eosinophilic, lipid depleted
cells intermixed with lipid-laden cells
Most important clinical consequences: Hypertension
Long term effects are cardiovascular compromise, stroke, MI,
“Nonclassic” Or Late-Onset Adrenogenitalism
hypokalemia
Hypertension – the most important clinical consequence of More common than the classic patterns
hyperaldosteronism (most common cause of secondary Partial deficiency in 21-hydroxylase function → later onset
hypertension) Virtually asymptomatic or have mild manifestations, such as
Promotes Na+ reabsorption through its effects on the hirsutism, acne, and menstrual irregularities.
mineralocorticoid receptor → ↑ Cardiac output Diagnosis: Demonstration of biosynthetic defects in
Long term effects steroidogenesis.
Cardiovascular compromise (LV hypertrophy, ↓diastolic
volumes) Salt-Wasting (“Classic”) Adrenogenitalism
Increased risk of stroke and myocardial infarction Inability to convert progesterone into deoxycorticosterone
Hypokalemia – renal potassium wasting as sodium is because of a total lack of 21-hydroxylase
reabsorbed Virtually no synthesis of mineralocorticoids
Previously a mandatory feature but has changed due to Blockage in the conversion of hydroxyprogesterone into
increasing numbers of normokalemic patients deoxycortisol resulting in cortisol deficiency
Hypokalemia is common but not pathognomonic Usually presents soon after birth, because in utero, the
electrolytes and fluids can be maintained by the maternal
Treatment kidneys.
Therapy varies according to cause Males are generally unrecognized at birth but come to clinical
Adenomas – surgical excision attention 5 - 15 days later because of some salt-losing crisis
Bilateral hyperplasia – medical intervention (aldosterone Salt wasting, hyponatremia, and hyperkalemia, which induce
antagonist, e.g. spironolactone) acidosis, hypotension, cardiovascular collapse, and possibly
Secondary hyperaldosteronism – correcting the underlying death
cause stimulating the RAAS
Simple Virilizing Adrenogenitalism
Diagnosis Presenting as genital ambiguity, in ~1/3 patients with 21-
Primary hyperaldosteronism – elevated ratios of plasma hydroxylase deficiency.
aldosterone concentration to plasma renin activity Generate sufficient mineralocorticoid to prevent a salt-
wasting “crisis”
Patho Title of Lecture 4 of 5
Lowered glucocorticoid level fails to cause feedback inhibition of
ACTH secretion
Increased level of testosterone → progressive virilization
Clinical Course:
Determined by the specific enzyme deficiency and
abnormalities related to androgen excess, with or without
aldosterone and glucocorticoid deficiency
21-hydroxylase deficiency causes excessive androgenic
activity
Signs of masculinization in females
Clitoral hypertrophy & pseudohermaphroditism in
infants,
Oligomenorrhea, hirsutism, and acne in postpubertal
females.
In males, androgen excess is associated with:
Enlargement of the external genitalia (precocious
puberty) in prepubertal patients
Oligospermia in older males.
CAH should be suspected in any neonate with ambiguous
genitalia
CAH affects not only adrenal cortical enzymes but also
products synthesized in the medulla.
Low cortisol levels + adrenomedullary dysplasia = low
catecholamine secretion
Predisposing these individuals to hypotension and
circulatory collapse
Severe enzyme deficiency in infancy can be a life-
threatening condition with vomiting, dehydration, and salt
wasting
Diagnosis
Classic CAH can be detected during newborn screening
Non-classic CAH: demonstration of biosynthetic defects in
steroidogenesis.
Treatment
Individuals with CAH – Tx is exogenous glucocorticoids
Suppresses ACTH levels → decrease the excessive
synthesis of the steroid hormones
Salt-wasting variants of CAH – Tx is mineralocorticoid
supplementation
Figure X. Pathogenesis of adrenogenital syndromes – 21-hydroxylase
deficiency is highlighted in red “blocks”
Patho Title of Lecture 5 of 5
You might also like
- Virology MnemonicsDocument2 pagesVirology MnemonicsReg Lagarteja100% (3)
- Clinical Therapeutics Oral Revalida Review Notes: CASE 1: Dr. Abraham Daniel C. CruzDocument31 pagesClinical Therapeutics Oral Revalida Review Notes: CASE 1: Dr. Abraham Daniel C. CruzNoreenNo ratings yet
- Chapters 1 and 2 (Harpers Biochemistry)Document16 pagesChapters 1 and 2 (Harpers Biochemistry)Joy Karen DamasigNo ratings yet
- Worksheet #3 Differential White Blood Cell CountDocument5 pagesWorksheet #3 Differential White Blood Cell CountROMAH JANE MENDOZANo ratings yet
- Anti-Anemia and Hematopoietic Growth FactorsDocument8 pagesAnti-Anemia and Hematopoietic Growth FactorsIsabel CastilloNo ratings yet
- Os 206: Pe of The Abdomen - ©spcabellera, Upcm Class 2021Document4 pagesOs 206: Pe of The Abdomen - ©spcabellera, Upcm Class 2021Ronneil BilbaoNo ratings yet
- Pharmacology Trans 1Document4 pagesPharmacology Trans 1GLOMARIE DE GUZMANNo ratings yet
- Chap. 18 GuytonDocument4 pagesChap. 18 GuytonMarian Joyce Princess Yuque100% (2)
- Clinical Case For SGD1Document1 pageClinical Case For SGD1Roselle AbrazaldoNo ratings yet
- NucleotidesDocument6 pagesNucleotidesDianne Ignacio100% (1)
- Migs (With Summary) +paoDocument6 pagesMigs (With Summary) +paoMigs MedinaNo ratings yet
- Biostatistics and Epidemiology: BioepiDocument25 pagesBiostatistics and Epidemiology: BioepiMaria Lana Grace DiazNo ratings yet
- Pleomorphic Adenoma AmeloblastomaDocument65 pagesPleomorphic Adenoma AmeloblastomaWilliam Tan CebrianNo ratings yet
- (P) Ana - Uerm 2Document11 pages(P) Ana - Uerm 2Aj BrarNo ratings yet
- MLS - HISTOLOGY Lec - M1-UNIT1 (Introduction To Histology)Document16 pagesMLS - HISTOLOGY Lec - M1-UNIT1 (Introduction To Histology)ataraNo ratings yet
- Muscle of Shoulder and Arm PDFDocument2 pagesMuscle of Shoulder and Arm PDFEdreyn DellosaNo ratings yet
- Physiology 100q.with TopicsDocument11 pagesPhysiology 100q.with TopicsdocaliNo ratings yet
- Trans of Harper's Illustrated Biochemistry CH 32Document3 pagesTrans of Harper's Illustrated Biochemistry CH 32Richelle Dianne Ramos-Giang100% (1)
- SGD 4 - Skeletal Muscle PhysiologyDocument3 pagesSGD 4 - Skeletal Muscle PhysiologyKriska Noelle0% (1)
- Tips For Ust Med FreshmenDocument3 pagesTips For Ust Med FreshmenFred EvidorNo ratings yet
- MBR 2019 - CNS Pathology HandoutDocument6 pagesMBR 2019 - CNS Pathology HandoutNica Lopez FernandezNo ratings yet
- Boards - Gen AnaDocument26 pagesBoards - Gen AnaJoyce EbolNo ratings yet
- Neuroanatomy SamplexDocument2 pagesNeuroanatomy SamplexMineTagraNo ratings yet
- PhysiologicDocument50 pagesPhysiologicRochelle Joyce AradoNo ratings yet
- Liver - RobbinsDocument25 pagesLiver - Robbinssarguss14100% (2)
- Cestodes 2020Document10 pagesCestodes 2020CDNo ratings yet
- Chapter 11 Blood Vessels 8th Ed NotesDocument7 pagesChapter 11 Blood Vessels 8th Ed NotesKyle Christopher SiaNo ratings yet
- Tissues Practical ExamDocument95 pagesTissues Practical ExamMarch AthenaNo ratings yet
- PHYSIO Samplex Motor SystemDocument42 pagesPHYSIO Samplex Motor SystemMelissa SalayogNo ratings yet
- Biochemistry QsDocument48 pagesBiochemistry QsFaisal AshiqNo ratings yet
- Lab Activity #1Document5 pagesLab Activity #1Charmaine RosalesNo ratings yet
- PHYSIO Prelims SamplexDocument11 pagesPHYSIO Prelims SamplexCarlos NiñoNo ratings yet
- REV Micro HSB RemedsDocument16 pagesREV Micro HSB RemedsPatricia HariramaniNo ratings yet
- Urine Preservatives - SLUDocument2 pagesUrine Preservatives - SLUShana Flame Haze0% (1)
- Biochemistry Review Notes: MembraneDocument18 pagesBiochemistry Review Notes: MembraneRanielle Samson100% (1)
- Chapter 34 Guyton and HallDocument5 pagesChapter 34 Guyton and Hallg_komolafeNo ratings yet
- 1.02 - Hemostasis, Surgical Bleeding and TransfusionsDocument11 pages1.02 - Hemostasis, Surgical Bleeding and TransfusionsPhilip Patrick LeeNo ratings yet
- Clinical MarasmusDocument27 pagesClinical MarasmusHANNo ratings yet
- Development of The Digestive SystemDocument90 pagesDevelopment of The Digestive Systemyusrah mukhtarNo ratings yet
- Chapter 20Document6 pagesChapter 20g_komolafeNo ratings yet
- 3.5 PHARMA ANTI MYCOBACTERIAL AGENTSpdfDocument18 pages3.5 PHARMA ANTI MYCOBACTERIAL AGENTSpdfJanet SantosNo ratings yet
- Patho A 1. 3 Inflammation and Repair (Dy-Quiangco, 2015)Document13 pagesPatho A 1. 3 Inflammation and Repair (Dy-Quiangco, 2015)kristineNo ratings yet
- Trans 1 - Cells As A Unit of Health and DiseaseDocument12 pagesTrans 1 - Cells As A Unit of Health and DiseaseCedrick BunaganNo ratings yet
- Biomphalaria&Aus Tralorbis Onchomelania Bulinus&PhysopsisDocument4 pagesBiomphalaria&Aus Tralorbis Onchomelania Bulinus&PhysopsisOlib OlieNo ratings yet
- Gastrointestinal PhysiologyDocument134 pagesGastrointestinal Physiologyapi-19916399100% (1)
- Practicals Histology Prelims ADocument36 pagesPracticals Histology Prelims AShaylla BretañaNo ratings yet
- Harper's Illustrated Biochemistry CH 35Document11 pagesHarper's Illustrated Biochemistry CH 35Richelle Dianne Ramos-Giang100% (1)
- 5 Hematologic-ExaminationsDocument12 pages5 Hematologic-ExaminationsMark Jireck AndresNo ratings yet
- Histology Model ADocument7 pagesHistology Model AOzgan SüleymanNo ratings yet
- Urinary System Disorders Practice Quiz #1 (50 Questions)Document26 pagesUrinary System Disorders Practice Quiz #1 (50 Questions)Emy TandinganNo ratings yet
- Chapter 22 Bone and Joints (2nd Edition)Document23 pagesChapter 22 Bone and Joints (2nd Edition)Abhi KarmakarNo ratings yet
- Bio StatisticsDocument28 pagesBio StatisticsBani AbdullaNo ratings yet
- CC Lab 6 TransesDocument6 pagesCC Lab 6 TransesCiara PamonagNo ratings yet
- Ust Anatomy Mock 2015-1 PDFDocument7 pagesUst Anatomy Mock 2015-1 PDFJanna Janoras ŰNo ratings yet
- 100 Questions Micro-Gross HSBDocument9 pages100 Questions Micro-Gross HSBfilchibuffNo ratings yet
- Systemic Response To InjuryDocument5 pagesSystemic Response To InjuryJohn Christopher LucesNo ratings yet
- Anatomy LE2 Samplex 2017BDocument7 pagesAnatomy LE2 Samplex 2017BHanako Sasaki AranillaNo ratings yet
- AdrenalDocument9 pagesAdrenalNada MuchNo ratings yet
- Surrene RobbinsDocument16 pagesSurrene RobbinsAndrea PescosolidoNo ratings yet
- Cushing's SyndromeDocument9 pagesCushing's Syndromemuzamiljoyia07No ratings yet
- Microbiome Impact On Metabolism and Function of Sex, Thyroid, Growth and Parathyroid Hormones 2015Document13 pagesMicrobiome Impact On Metabolism and Function of Sex, Thyroid, Growth and Parathyroid Hormones 2015Brenda FolkNo ratings yet
- Analysing The Levels of Various Biochemical Markers (T3, T4, and TSH) in Iraqi Patients With Thyroid ProblemsDocument10 pagesAnalysing The Levels of Various Biochemical Markers (T3, T4, and TSH) in Iraqi Patients With Thyroid ProblemsCentral Asian StudiesNo ratings yet
- Human Reproduction - Part 1Document22 pagesHuman Reproduction - Part 1NickOoPandeyNo ratings yet
- 5 Hpe Axis Sum21 StudentDocument7 pages5 Hpe Axis Sum21 StudentRAQUEL WINECKNo ratings yet
- Risk Factor HyperthyroidDocument3 pagesRisk Factor HyperthyroidTri NataNo ratings yet
- Hipo HiperthyroidDocument49 pagesHipo HiperthyroidMuhammad Bilal Bin AmirNo ratings yet
- Adrenal Function TestsDocument103 pagesAdrenal Function TestsPriyanshu MandalNo ratings yet
- نسخة ENDO-17-2Document16 pagesنسخة ENDO-17-2Sami MdNo ratings yet
- Integumentary System: Student Learning ObjectivesDocument3 pagesIntegumentary System: Student Learning ObjectivesYesita AdamyNo ratings yet
- Endocrine Ed WorksheetDocument2 pagesEndocrine Ed WorksheetCathleen Shane CarcabusoNo ratings yet
- IfU ATG1010engl, DT, Es-18062014 Ab Lot 016Document23 pagesIfU ATG1010engl, DT, Es-18062014 Ab Lot 016f.baxyNo ratings yet
- What Is HyperthyroidismDocument7 pagesWhat Is HyperthyroidismGelo LeañoNo ratings yet
- HPA Axis Terkait Dengan Gangguan Jiwa (Dr. Hadi) .PPSXDocument19 pagesHPA Axis Terkait Dengan Gangguan Jiwa (Dr. Hadi) .PPSXMbak RockerNo ratings yet
- Ovulation Induction Evidence Based GuidelinesDocument207 pagesOvulation Induction Evidence Based Guidelineswaleedali100% (3)
- Endocrine Glands PathologyDocument8 pagesEndocrine Glands Pathologychrisp7No ratings yet
- ANAPHY LAB Worksheet 8.1 Endocrine SystemDocument3 pagesANAPHY LAB Worksheet 8.1 Endocrine SystemKristine Lorainne DelamideNo ratings yet
- Chapter 34 The Endocrine SystemDocument39 pagesChapter 34 The Endocrine SystemCarl Agape Davis100% (3)
- Hypothalamo HypophyseDocument58 pagesHypothalamo Hypophyseyinuo94No ratings yet
- Thyroid Function TestsDocument1 pageThyroid Function TestsWail hassan ShahadaNo ratings yet
- Menstrual Cycle LP - 1st CODocument8 pagesMenstrual Cycle LP - 1st COEsther Mae Ann TrugilloNo ratings yet
- LEC 01 - Principles of EndocrinologyDocument44 pagesLEC 01 - Principles of EndocrinologyIoana Cozma100% (1)
- Amenorrhea, Hirsutism, VirilismDocument12 pagesAmenorrhea, Hirsutism, VirilismAly MorganNo ratings yet
- (03241750 - Acta Medica Bulgarica) Minimally Invasive Radiofrequency Ablation For Large Thyroid Toxic AdenomaDocument3 pages(03241750 - Acta Medica Bulgarica) Minimally Invasive Radiofrequency Ablation For Large Thyroid Toxic AdenomaTeodorNo ratings yet
- Lab 9 Endocrinology Atlas Edited For LabDocument6 pagesLab 9 Endocrinology Atlas Edited For LabWilliam DelphNo ratings yet
- Module 3Document20 pagesModule 3VENTURA, ANNIE M.No ratings yet
- What Is The Hypothalamus?Document4 pagesWhat Is The Hypothalamus?Jae Em DiestroNo ratings yet
- Tog 12665Document13 pagesTog 12665saeed hasan saeed100% (1)
- Endocrine System: Related Kidshealth LinksDocument8 pagesEndocrine System: Related Kidshealth LinksDianaCornejoNo ratings yet
- 4 - PASTEST 2019 For MRCP2-Dr - Hisham Alshamekh - EndocrineDocument763 pages4 - PASTEST 2019 For MRCP2-Dr - Hisham Alshamekh - EndocrineYS NateNo ratings yet
- Endocrine System Disorders Endocrine Disorders - Too Much GigantismDocument4 pagesEndocrine System Disorders Endocrine Disorders - Too Much GigantismDhea Angela A. CapuyanNo ratings yet