Professional Documents
Culture Documents
Titanium Dioxide Chemical and Technical Assessment First Draft Prepared by Paul M. Kuznesof, Ph.D. Reviewed by M.V. Rao, PH.D
Uploaded by
akshit patidarOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Titanium Dioxide Chemical and Technical Assessment First Draft Prepared by Paul M. Kuznesof, Ph.D. Reviewed by M.V. Rao, PH.D
Uploaded by
akshit patidarCopyright:
Available Formats
TITANIUM DIOXIDE
Chemical and Technical Assessment
First draft prepared by Paul M. Kuznesof, Ph.D.
Reviewed by M.V. Rao, Ph.D.
1. Summary
Titanium dioxide (INS no. 171; CAS no. 13463-67-7) is produced either in the anatase or rutile
crystal form. Most titanium dioxide in the anatase form is produced as a white powder, whereas
various rutile grades are often off-white and can even exhibit a slight colour, depending on the
physical form, which affects light reflectance. Titanium dioxide may be coated with small
amounts of alumina and silica to improve technological properties.
Commercial titanium dioxide pigment is produced by either the sulfate process or the chloride
process. The principal raw materials for manufacturing titanium dioxide include ilmenite
(FeO/TiO2), naturally occurring rutile, or titanium slag. Both anatase and rutile forms of
titanium dioxide can be produced by the sulfate process, whereas the chloride process yields the
rutile form.
Titanium dioxide can be prepared at a high level of purity. Specifications for food use currently
contain a minimum purity assay of 99.0%.
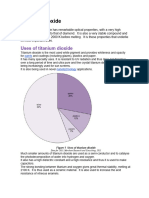
Titanium dioxide is the most widely used white pigment in products such as paints, coatings,
plastics, paper, inks, fibres, and food and cosmetics because of its brightness and high refractive
index (> 2.4), which determines the degree of opacity that a material confers to the host matrix.
When combined with other colours, soft pastel shades can be achieved. The high refractive
index, surpassed by few other materials, allows titanium dioxide to be used at relatively low
levels to achieve its technical effect.
The food applications of titanium dioxide are broad. US regulations authorize its use as a colour
additive in foods in general at levels not to exceed 1%. The European Union also permits its use
in foods, in general, with some specified exceptions, at quantum satis levels. India restricts its
uses to chewing gum and bubble gum at not more than 1% and to powdered concentrate mixes
for fruit beverage drinks not to exceed 100 mg/kg. Japan lists its use as a food colour without
limitation, other than specifying certain foods in which it is not permitted. Finally, titanium
dioxide is listed in Table 3 of the Codex General Standard for Food Additives, which lists
additives that may be used in food, in general, unless otherwise specified, in accordance with
GMP.
2. Description
Titanium dioxide or Titanium (IV) oxide (TiO2, Formula Weight 79.88).
CAS no. 13463-67-7.
Synonyms: INS no. 171; Titania; CI Pigment White 6; CI (1975), no. 77891.
Natural titanium dioxide exists in nature in one of three crystalline forms, the two most
important of which are anatase (CAS no. 1317-70-0) and rutile (CAS no. 1317-80-2), the third
being brookite (12188-41-9). Although these minerals are essentially pure titanium dioxide,
they do not appear white, because of the presence of impurities, such as iron, chromium, or
vanadium, which darken them. Rutile is the thermodynamically stable form of titanium dioxide;
Titanium dioxide (CTA) 2006 - Page 1(8)
anatase rapidly transforms to rutile above 700o. Rutile melts between 1830o and 1850o (Kirk-
Othmer, 1997; Kirk-Othmer, 2006).
Commercial titanium dioxide is generally marketed as a white to slightly coloured amorphous
powder. A platelet form is also manufactured. Most titanium dioxide in the anatase form is
produced as a white powder, whereas various rutile grades are often off-white and can even
exhibit a slight colour, depending on the physical form affecting light reflectance. Titanium
dioxide may be coated with small amounts of alumina and silica to improve technological
properties. Such coatings can prevent possible reactions between the highly reactive surfaces of
the extremely fine titanium dioxide crystals and the matrix in which the pigment is dispersed
and they can improve the dispersion of the titanium dioxide in the host matrix (Kirk-Othmer,
2006).
Titanium dioxide is insoluble in water, hydrochloric acid, dilute sulfuric acid, and organic
solvents. It dissolves slowly in hydrofluoric acid and hot concentrated sulfuric acid. It is almost
insoluble in aqueous alkaline media (Kirk-Othmer, 1997).
3. Method of manufacture
Commercial titanium dioxide pigment is produced by either the sulfate process or the chloride
process. Because of significant environmental and cost issues associated with the sulfate
process, most new manufacturing capacity is based on the chloride process. Older
manufacturing plants that used the sulfate process have had to modify their processes to
accommodate stricter environmental requirements by recycling waste acids and roasting metal
sulfates to recover sulfur trioxide (Kirk-Othmer, 1997; DeMerlis, 2005).
The principal raw materials for manufacturing titanium dioxide include ilmenite (FeO/TiO2),
naturally occurring rutile, and titanium slag. The last is produced by removing the iron from
ilmenite by reduction with coke at 1200-1600o. At these temperatures, the iron oxide is reduced
to the metal, which melts and separates from the formed titanium-containing slag, which is 70-
75% titanium dioxide (Kirk-Othmer, 2006).
Sulfate process (Kirk-Othmer, 1997; Kirk-Othmer, 2006; DeMerlis, 2005)
Both anatase and rutile grades of titanium dioxide can be produced by the sulfate process,
depending on particular processing conditions. Briefly, ilmenite or ilmenite and titanium slag is
digested with sulfuric acid and the product is diluted with water or dilute acid. Most of the
titanium dioxide from the ore is solubilized as a titanium oxo-sulfate and iron is present in its
+II oxidation state. The resulting liquor is clarified by sedimentation to remove insoluble
residues such as silica. Iron is removed by crystallization as its sulfate salt (FeSO4•7H2O),
followed by filtration.
To produce the anatase form of the titanium dioxide, a small portion of the clarified liquor is
neutralized with alkali to produce anatase microcrystals. These microcrystals are then
introduced into the mother liquor, which is then hydrolysed under carefully controlled
conditions to produce crystals of anatase. These are subsequently filtered, washed, calcined, and
micronized. During calcination, the final temperature reaches about 800-850o.
To produce the rutile form of the titanium dioxide, the clarified liquor is hydrolyzed in the
presence of a specially prepared rutile seeding agent obtained by neutralizing a small portion of
the mother liquor in the presence of hydrochloric acid or some other monohydric acid. Formed
crystals are filtered, washed and calcined at temperatures between 900 and 930o, and
micronized.
Titanium dioxide (CTA) 2006 - Page 2(8)
Chloride process (Kirk-Othmer, 1997; Kirk-Othmer, 2006; DeMerlis, 2005)
The chloride process yields the rutile form of titanium dioxide. At temperatures between 800
and 1200o, chlorine is reacted in a fluidized bed reactor with a titanium-containing mineral, e.g.,
mineral rutile (which is not readily attacked by sulfuric acid), under reducing conditions
(presence of coke) to form anhydrous titanium (IV) chloride. Purification of the anhydrous
tetrachloride requires separation by fractional condensation. Conversion of the tetrachloride to
titanium dioxide may be accomplished by either direct thermal oxidation or reaction with steam
in the vapour phase at temperatures in the range of 900-1400o. A minor amount of aluminium
chloride is generally added to promote formation of the rutile form. The titanium dioxide is
washed, calcined, and packaged.
Alternatively, the titanium-containing mineral can be reacted with concentrated hydrochloric
acid to form solutions of titanium (IV) chloride which are then further purified. Hydrolysis of
the tetrachloride will yield the dioxide which is filtered off, washed, calcined, and packaged
(DeMerlis, 2005).
Titanium dioxide/platelet form (EFSA, 2004)
A platelet form of titanium dioxide (rutile) can be produced by first coating the surface of mica
(i.e., potassium aluminum silicate) platelets, which act as templates, with the titanium dioxide
(rutile). The titanium dioxide-coated mica nacreous pigment is then subjected to an extractive
dissolution in acid followed by an extractive dissolution in alkali. All of the mica is removed
during this process and the resulting product is a platelet form of rutile titanium dioxide. This
product cannot be obtained from anatase titanium dioxide as a starting material.
The specific properties of the pigment are determined by controlling the thickness of the
titanium dioxide layer and the coating process used to coat the mica substrate. The thickness of
the rutile titanium dioxide coated on the mica determines the interference colour of the final
product. The resulting platelet titanium dioxide contains low levels of impurities comparable to
other standard pigment grades of titanium dioxide typically used in the food industry.
4. Characterization
4.1 Composition
Titanium dioxide can be prepared at a high level of purity. Specifications for food use currently
contain a minimum purity assay of 99.0% (FCC, 2003; Japan, 2000; JECFA, 2006). Maximum
limits for Loss on Drying (Japan, 2000; JECFA, 2006) and Loss on Ignition (FCC, 2003; Japan,
2000; JECFA, 2006) have also been established.
4.2 Possible impurities
The possible impurities in titanium dioxide arise from the impurities in the ores and solvents
used in the manufacturing process. The following Table indicates the maximum limits specified
in the JECFA and Food Chemicals Codex (FCC) monographs for various impurities.
Maximum Specified Limits for Impurities in Titanium Oxide
Impurity JECFA (2006) FCC (2003) Japan (2000)
Aluminium oxide/silicon dioxide 2% 2.0% ---
Acid-soluble substances 0.5% (1.5% for products 0.5% 0.50%
containing alumina or silica)
Water-soluble matter 0.5% 0.3% 0.25%
Antimony 2 mg/kg 1 mg/kg (a)
Arsenic 1 mg/kg 2 mg/kg 1.3 mg/kg as As2O3
Cadmium 1 mg/kg --- (a)
Lead 10 mg/kg 10 mg/kg (a)
Mercury 1 mg/kg 1 mg/kg (a)
(a) 10 mg/kg total Heavy metals (as lead).
Titanium dioxide (CTA) 2006 - Page 3(8)
4.3 Analytical methods
The FCC, Japanese, and JECFA specifications monographs for titanium dioxide (FCC, 2003;
Japan, 2000; JECFA, 2006), as well as the volume on general methods in the Combined
Compendium of Specifications (JECFA, 2006a), provide analytical methods for all tests
indicated.
5.Functional uses
Titanium dioxide is the most widely used white pigment in products such as paints, coatings,
plastics, paper, inks, fibres, and food and cosmetics because of its brightness and high refractive
index (> 2.4), which determines the degree of opacity that a material confers to the host matrix.
The high refractive index, surpassed by few other materials, allows titanium dioxide to be used
at relatively low levels to achieve its technical effect (CERAM, 2006; Wikipedia, 2006)).
Titanium dioxide is highly effective as a whitener for confectionary, baked goods, cheeses,
icings, toppings, and food supplements. When combined with other colours, soft pastel shades
can be achieved. As titanium dioxide is not water-soluble, applications require dispersion using
vehicles such as food oils, propylene glycol, sugar syrup, or water with select thickeners
(Gerdes, 2004; Vaughn, 2006).
The Codex General Standard for Food Additives (Table 3 - Additives Permitted for Use in Food
in General, Unless Otherwise Specified, in Accordance with GMP) contains a provision for the
use of titanium dioxide in food (Codex, 2005). The acceptable food categories of this standard
(the GSFA) corresponding to general use are reproduced in ANNEX 1, taken from the GSFA
on-line data base (Codex, 2005a). Maximum numerical levels of use are not specified for Table
3 additives. GMP (Good Manufacturing Practices) is understood to mean that levels of use
should be no more than necessary to achieve the intended technical effect. (Caution: The entries
in ANNEX 1 do not mean that titanium dioxide is being used in all the listed food categories.)
The United States Food and Drug Administration (FDA) has authorized the use of titanium
dioxide in food, in general, at a limit not to exceed 1% by weight of the food. Silicon dioxide
and aluminium oxide may be used with the titanium dioxide as “dispersing aids” at levels not to
exceed 2% by weight, singly or in combination, of the titanium dioxide (FDA, 2005). In the
USA, actual uses of titanium dioxide in food supplements and hard and soft panned candies
have been reported to be as high as 1%. Uses in icings, chewing gums, starch-molded
confectionary, and baked goods typically range from 0.02% to 2% and in savory snack foods
from 0.05% to 0.4% (Vaughn, 2006).
India restricts the uses of titanium dioxide to chewing gum and bubble gum with a level not to
exceed 1% (India, 2004) and to powdered concentrate mixes for fruit beverage drinks not to
exceed 100 mg/kg (India 2004a).
Japan lists its use as a food colour without limitation, other than specifying certain foods in
which it is not permitted (Japan, 2000).
The European Union lists titanium dioxide for use in colouring food, in general, except for
certain specified foodstuffs, at quantum satis levels (EU, 1994).
A recent report by the European Food Safety Authority, EFSA, notes that the rutile form of
titanium dioxide, as platelets, is currently used in aqueous film coating systems for commercial
confectionery products in the United States. EFSA states that the platelet form of the additive is
being evaluated by the United States food industry for use in cookies, baked goods, pretzels and
other salted snacks, and in confectionery products (EFSA, 2004).
Titanium dioxide (CTA) 2006 - Page 4(8)
6. Reactions and fate in foods
Given its insolubility in water, hydrochloric acid, dilute sulfuric acid, and organic solvents,
titanium dioxide is not expected to react with components of food. Its functionality depends upon
its inertness within a food matrix. In fact, in 1969 JECFA (JECFA, 1970) “decided not to
establish a limit on intake of titanium dioxide since the evidence indicates that it is free from toxic
effects on account of its insolubility and inertness. The intake in food would be limited by good
manufacturing practice.”
7. References
CERAM (2006). CERAM Research – http://www.azom.com/details.asp?ArticleID=1179 –
accessed 9 March.
Codex (2005). CX/STAN 192-1995, rev. 2005, Codex General Standard for Food Additives
(GSFA). Codex Alimentarius Commission, FAO, Rome.
Codex (2005a). CX/STAN 192-1995, rev. 2005, Codex General Standard for Food Additives
(GSFA) Online Database: http://www.codexalimentarius.net/gsfaonline/index.html – accessed
10 March 2006.
DeMerlis, C.C. (2005). Colorcon letter to A. Wennberg, FAO-JECFA Secretary, 6 December
and accompanying dossier prepared for the 67th meeting (June 2006) of the Joint FAO/WHO
Expert Committee on Food Additives.
EFSA (2004). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids
and materials in Contact with Food (European Food Safety Authority) on a request from the
Commission related to the safety in use of rutile titanium dioxide as an alternative to the
presently permitted anatase form. Question N° EFSA-Q-2004-103. Adopted on 7 December
2004. The EFSA Journal, 163:1-12.
EU (1994). European Parliament and Council Directive on Colours, 94/36/EC. Official J.
Europ. Comm., L237: 13-29.
FCC (2003). Titanium dioxide. In Food Chemicals Codex, 5th ed, National Academies Press,
Washington, DC.
FDA (2005). Titanium dioxide. In The United States Code of Federal Regulations, Title 21,
Section 73.575, Office of the Federal Register, Washington, DC.
Gerdes, S. (2004). Perusing the Food Color Palette. In Food Product Design (December 2004)
(http://www.foodproductdesign.com/archive/2004/1204DE.html), Virgo Publishing,
Northbrook, Illinois.
India (2004). Chewing gum and Bubble gum. In The Prevention of Food Adulteration Act and
Rules, 1955, Appendix B, Section A.25.02.01, Directorate General of Health Services, Ministry
of Health, New Deli.
India (2004a). Powdered concentrate mixes for fruit beverage drinks. In The Prevention of
Food Adulteration Act and Rules, 1955, Appendix C, Table 3, Directorate General of Health
Services, Ministry of Health, New Deli.
Japan (2000). Titanium dioxide. In Japanese Specifications and Standards for Food Additives,
7th ed, Ministry of Health and Welfare, Tokyo.
Titanium dioxide (CTA) 2006 - Page 5(8)
JECFA (1970). Thirteenth Report of the Joint FAO/WHO Expert Committee on Food
Additives (Rome, 27 May - 4 June 1969). WHO Technical Report Series No. 445 and FAO
Nutrition Meetings Report Series No. 46, WHO, Geneva.
JECFA (2006). Titanium dioxide. In Combined Compendium of Food Additive Specifications,
Vol. 3, FAO, Rome.
JECFA (2006a). Combined Compendium of Food Additive Specifications, Vol. 4, FAO, Rome.
Kirk-Othmer (1997). Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed, Vol. 24. John
Wiley and Sons, New York, pp. 233-250.
Kirk-Othmer (2006). Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed, Vol. 19. John
Wiley and Sons, New York, pp. 387-393.
Vaughn, A. (2006). Colorcon email to P.M. Kuznesof (16 May) in preparation for the 67th
meeting (June 2006) of the Joint FAO/WHO Expert Committee on Food Additives.
Wikipedia (2006). http://en.wikipedia.org/wiki/Titanium_dioxide - accessed 9 March.
Titanium dioxide (CTA) 2006 - Page 6(8)
ANNEX 1
Provisions for the Use of Titanium Dioxide
According to Table 3 of the Codex General Standard for Food Additives
(CX/STAN 192-1995, rev. 2005)
GSFA Table 3 Provisions
Titanium Dioxide is a food additive that is included in Table 3, and as such may be used in the following foods under the
conditions of good manufacturing practices (GMP) as outlined in the Preamble of the Codex GSFA. Note that food categories
listed in the Annex to Table 3 were excluded accordingly.
Number Food Category
01.1.2 Dairy-based drinks, flavoured and/or fermented (e.g., chocolate milk, cocoa, eggnog,
drinking yoghurt, whey-based drinks)
01.3 Condensed milk and analogues (plain)
01.4.3 Clotted cream (plain)
01.4.4 Cream analogues
01.5 Milk powder and cream powder and powder analogues (plain)
01.6 Cheese and analogues
01.7 Dairy-based desserts (e.g., pudding, fruit or flavoured yoghurt)
01.8 Whey and whey products, excluding whey cheeses
02.2.1.2 Margarine and similar products
02.2.1.3 Blends of butter and margarine
02.2.2 Emulsions containing less than 80% fat
02.3 Fat emulsions maily of type oil-in-water, including mixed and/or flavoured products
based on fat emulsions
02.4 Fat-based desserts excluding dairy-based dessert products of food category 01.7
03.0 Edible ices, including sherbet and sorbet
04.1.2 Processed fruit
04.2.2.2 Dried vegetables (including mushrooms and fungi, roots and tubers, pulses and legumes,
and aloe vera), seaweeds, and nuts and seeds
04.2.2.3 Vegetables (including mushrooms and fungi, roots and tubers, pulses and legumes, and
aloe vera) and seaweeds in vinegar, oil, brine, or soy sauce
04.2.2.4 Canned or bottled (pasteurized) or retort pouch vegetables (including mushrooms and
fungi, roots and tubers, pulses and legumes, and aloe vera), and seaweeds
04.2.2.5 Vegetable (including mushrooms and fungi, roots and tubers, pulses and legumes, and
aloe vera), seaweed, and nut and seed purees and spreads (e.g., peanut butter)
04.2.2.6 Vegetable (including mushrooms and fungi, roots and tubers, pulses and legumes, and
aloe vera), seaweed, and nut and seed pulps and preparations (e.g., vegetable desserts and
sauces, candied vegetables) other than food category 04.2.2.5
04.2.2.8 Cooked or fried vegetables (including mushrooms and fungi, roots and tubers, pulses and
legumes, and aloe vera), and seaweeds
05.0 Confectionery
06.3 Breakfast cereals, including rolled oats
06.4.3 Pre-cooked pastas and noodles and like products
06.5 Cereal and starch based desserts (e.g., rice pudding, tapioca pudding)
06.6 Batters (e.g., for breading or batters for fish or poultry)
06.7 Pre-cooked or processed rice products, including rice cakes (Oriental type only)
06.8 Soybean products (excluding soybean products of food category 12.9 and fermented
soybean products of food category 12.10)
07.0 Bakery wares
08.2 Processed meat, poultry, and game products in whole pieces or cuts
08.3 Processed comminuted meat, poultry, and game products
Titanium dioxide (CTA) 2006 - Page 7(8)
Number Food Category
08.4 Edible casings (e.g., sausage casings)
09.3 Semi-preserved fish and fish products, including mollusks, crustaceans, and echinoderms
09.4 Fully preserved, including canned or fermented fish and fish products, including
mollusks, crustaceans, and echinoderms
10.2.3 Dried and/or heat coagulated egg products
10.3 Preserved eggs, including alkaline, salted, and canned eggs
10.4 Egg-based desserts (e.g., custard)
11.6 Table-top sweeteners, including those containing high-intensity sweeteners
12.2.2 Seasonings and condiments
12.3 Vinegars
12.4 Mustards
12.5 Soups and broths
12.6 Sauces and like products
12.7 Salads (e.g., macaroni salad, potato salad) and sandwich spreads excluding cocoa- and
nut-based spreads of food categories 04.2.2.5 and 05.1.3
12.8 Yeast and like products
12.9 Protein products
12.10 Fermented soybean products
13.3 Dietetic foods intended for special medical purposes (excluding products of food category
13.1)
13.4 Dietetic formulae for slimming purposes and weight reduction
13.5 Dietetic foods (e.g., supplementary foods for dietary use) excluding products of food
categories 13.1 - 13.4 and 13.6
13.6 Food supplements
14.1.1.2 Table waters and soda waters
14.1.4 Water-based flavoured drinks, including "sport," "energy," or "electrolyte" drinks and
particulated drinks
14.2.1 Beer and malt beverages
14.2.2 Cider and perry
14.2.4 Wines (other than grape)
14.2.5 Mead
14.2.6 Distilled spirituous beverages containing more than 15% alcohol
14.2.7 Aromatized alcoholic beverages (e.g., beer, wine and spirituous cooler-type beverages,
low alcoholic refreshers)
15.0 Ready-to-eat savouries
16.0 Composite foods - foods that could not be placed in categories 01 - 15
Titanium dioxide (CTA) 2006 - Page 8(8)
You might also like
- TiO2 Titanium Dioxide Extraction Production Project PresentationDocument48 pagesTiO2 Titanium Dioxide Extraction Production Project Presentationkaranved780% (5)
- Powerpoint Presentation On Extraction of TitaniumDocument31 pagesPowerpoint Presentation On Extraction of TitaniumLuis Sahoo90% (10)
- Project ReportDocument53 pagesProject ReportChunchu Anil100% (1)
- Benelite Process For Upgradation of IllemniteDocument6 pagesBenelite Process For Upgradation of Illemnitemadangk100% (1)
- J. Sheehan, Richard - Ullmann's Encyclopedia of Industrial Chemistry - Terephthalic Acid, Dimethyl Terephthalate, and Isophthalic AcidDocument13 pagesJ. Sheehan, Richard - Ullmann's Encyclopedia of Industrial Chemistry - Terephthalic Acid, Dimethyl Terephthalate, and Isophthalic AcidNithya SethuganapathyNo ratings yet
- Micro TeachingDocument6 pagesMicro TeachingNirmaladevi SubramaniamNo ratings yet
- Lakheri Cement Works: Report On Overhauling (Replacement of Internals) of Vrm-1 Main Gearbox 22-May-09 To 27-Jun-09Document47 pagesLakheri Cement Works: Report On Overhauling (Replacement of Internals) of Vrm-1 Main Gearbox 22-May-09 To 27-Jun-09sandesh100% (1)
- Production of Titanium DioxideDocument16 pagesProduction of Titanium Dioxidehaisamdo100% (1)
- Manufacture of Titanium DioxideDocument52 pagesManufacture of Titanium DioxideJayraj Dayma100% (1)
- New Microsoft PowerPoint PresentationDocument31 pagesNew Microsoft PowerPoint PresentationASHRAFUL KABIRNo ratings yet
- Titanium DioxideDocument18 pagesTitanium DioxidePrrinisha KanabathyNo ratings yet
- Uses of Titanium DioxideDocument5 pagesUses of Titanium DioxideAbdul RazzaqueNo ratings yet
- Hydrometallurgy: Scott Middlemas, Z. Zak Fang, Peng FanDocument7 pagesHydrometallurgy: Scott Middlemas, Z. Zak Fang, Peng FanmonisalesNo ratings yet
- Titaniun Dioxide Basic InformationDocument4 pagesTitaniun Dioxide Basic Informationalexandria.padilla11No ratings yet
- Life Cycle Assessment Comparison of Emerging and Traditional Titanium Dioxide Manufacturing ProcessesDocument11 pagesLife Cycle Assessment Comparison of Emerging and Traditional Titanium Dioxide Manufacturing ProcessesFelipe TavaresNo ratings yet
- Mono93 7Document84 pagesMono93 7Boopathi MechNo ratings yet
- Review Extractive Metallurgy Titanium - Daffa HandyanDocument6 pagesReview Extractive Metallurgy Titanium - Daffa HandyanDaffa HandyanNo ratings yet
- Sulfate Digestion Process For High Purity Tio From Titania SlagDocument6 pagesSulfate Digestion Process For High Purity Tio From Titania SlagQuang VANo ratings yet
- 8001 A New Process To Upgrade Ilmenite To Synthetic RutileDocument10 pages8001 A New Process To Upgrade Ilmenite To Synthetic RutileJeancarlo Salinas Diaz100% (1)
- 16-04-2020-1587032337-6 - Ijce-2. Ijce - Processing of Rutile Concentrates by FluorinationDocument18 pages16-04-2020-1587032337-6 - Ijce-2. Ijce - Processing of Rutile Concentrates by Fluorinationiaset123No ratings yet
- HAZMAT Articles - Titanium TetrachlorideDocument2 pagesHAZMAT Articles - Titanium TetrachlorideRadko BankrasNo ratings yet
- Environmental Problems of Finely Dispersed Titanium Dioxide ProductionDocument13 pagesEnvironmental Problems of Finely Dispersed Titanium Dioxide ProductionDharamNo ratings yet
- Oxide Titanium Ti O PigmentDocument10 pagesOxide Titanium Ti O Pigmentapi-27149699No ratings yet
- TitaniumDocument2 pagesTitaniumpotterhead1DNo ratings yet
- BecherDocument7 pagesBechervalholNo ratings yet
- A Literature Review of Titanium Metallurgical ProcessesDocument12 pagesA Literature Review of Titanium Metallurgical ProcessesMargarita CaceresNo ratings yet
- Molecular Formula: PropertiesDocument4 pagesMolecular Formula: PropertiesAngga YuniantoNo ratings yet
- Specialty ChemicalsDocument30 pagesSpecialty ChemicalsucheNo ratings yet
- Occurrence: /taɪ Teɪniə/ Oxide Titanium Pigment Ilmenite Rutile Anatase Paint Sunscreen Food Coloring E NumberDocument4 pagesOccurrence: /taɪ Teɪniə/ Oxide Titanium Pigment Ilmenite Rutile Anatase Paint Sunscreen Food Coloring E NumberVinod NairNo ratings yet
- Preparation of Highly Pure Thorium Nitrate Via Thorium Sulfate and Thorium PeroxideDocument4 pagesPreparation of Highly Pure Thorium Nitrate Via Thorium Sulfate and Thorium PeroxideGyan PrameswaraNo ratings yet
- Niobium Processing NotesDocument5 pagesNiobium Processing NotesMarto KarezNo ratings yet
- Formation and Characterization of Nano Sized Tio Powder by Sol-Gel MethodDocument9 pagesFormation and Characterization of Nano Sized Tio Powder by Sol-Gel MethoddmaheswariNo ratings yet
- Production of Titanium Tetrachloride (TiCl4) From Titanium Ores - A ReviewDocument49 pagesProduction of Titanium Tetrachloride (TiCl4) From Titanium Ores - A Reviewrazor75apNo ratings yet
- DL Metal HydridesDocument20 pagesDL Metal HydridesGoutam Gottumukkala100% (1)
- Nano Titania PowerPoint Presentationد رضويDocument40 pagesNano Titania PowerPoint Presentationد رضويArilu2010No ratings yet
- Schm312 NotesDocument97 pagesSchm312 NotesSandile SynthaxError Mabika100% (1)
- Manufacturing Fine TiO2 Particles by Chloride ProcessDocument2 pagesManufacturing Fine TiO2 Particles by Chloride ProcessDiahAyuSafitriNo ratings yet
- Titanium DioxideDocument18 pagesTitanium DioxideUnity EfejeneNo ratings yet
- Trichloroethylene ThesisDocument62 pagesTrichloroethylene Thesisessakkirajm19902116100% (1)
- 10.1016 J.jallCOM.2005.10.048 Extraction of Titanium From Different Titania Precursors by The FFC Cambridge ProcessDocument9 pages10.1016 J.jallCOM.2005.10.048 Extraction of Titanium From Different Titania Precursors by The FFC Cambridge ProcessHooman BaghbanNo ratings yet
- SSRN Id4553127Document5 pagesSSRN Id4553127ghinasaleh97No ratings yet
- Group Acetic Acid PresentationDocument24 pagesGroup Acetic Acid PresentationNatko47No ratings yet
- Titanium Tetrachloride Production by The ChlorideDocument8 pagesTitanium Tetrachloride Production by The ChlorideMargarita CaceresNo ratings yet
- Kroll ProcessDocument5 pagesKroll ProcessNavarro SalgadoNo ratings yet
- Gujarat Technological University: Bachelor of EngineerDocument12 pagesGujarat Technological University: Bachelor of EngineerChemical Engg.No ratings yet
- Production of Acetic Acid by Methanol CarbonalyzationDocument139 pagesProduction of Acetic Acid by Methanol CarbonalyzationNoman Aslam100% (1)
- Ullmann's Enc. of Industrial ChemistryDocument13 pagesUllmann's Enc. of Industrial ChemistryEstela HirataNo ratings yet
- Sulfuric Acid & Top 20 ChemicalsDocument2 pagesSulfuric Acid & Top 20 ChemicalsavwnashNo ratings yet
- Manufactures in Industry and Chemical For ConsumerDocument22 pagesManufactures in Industry and Chemical For ConsumerMuhammad DanishNo ratings yet
- Alkaline Process 2019Document9 pagesAlkaline Process 2019Major TomNo ratings yet
- Iluka-Zircon and OtherDocument13 pagesIluka-Zircon and OtherArief MahdiNo ratings yet
- Golongan Ivb - English VersionDocument20 pagesGolongan Ivb - English VersionAriska AndrainiNo ratings yet
- Nitric Acid and Aluminium-Sodium: Report OnDocument31 pagesNitric Acid and Aluminium-Sodium: Report OnDisha GardiNo ratings yet
- Chapter 57 - Titanium - 2015 - Handbook On The Toxicology of MetalsDocument10 pagesChapter 57 - Titanium - 2015 - Handbook On The Toxicology of MetalsChanWingSanNo ratings yet
- NaNO3 SolutionDocument8 pagesNaNO3 SolutionPham Thanh LyNo ratings yet
- Synthesis of Tin Oxide Nanoparticles by SolDocument2 pagesSynthesis of Tin Oxide Nanoparticles by SolMyo Wai AungNo ratings yet
- Presentation of Titanium DioxideDocument4 pagesPresentation of Titanium Dioxidem_turabNo ratings yet
- Evaluation of Titanium in Hydrochloric Acid SolutiDocument5 pagesEvaluation of Titanium in Hydrochloric Acid SolutiDalila SilveiraNo ratings yet
- Technical Report of Formic Acid PlantDocument6 pagesTechnical Report of Formic Acid PlantMuzzamilNo ratings yet
- Toluenediamine PDFDocument15 pagesToluenediamine PDFAmalia RizkaNo ratings yet
- Geometry Sparkcharts Geometry Sparkcharts: Book Review Book ReviewDocument3 pagesGeometry Sparkcharts Geometry Sparkcharts: Book Review Book ReviewAyman BantuasNo ratings yet
- Strategic ManagementDocument91 pagesStrategic ManagementAniket DEORENo ratings yet
- 6.1 Mean Median Mode and RangeDocument21 pages6.1 Mean Median Mode and RangeGilbert Guzman TurarayNo ratings yet
- 1 Scrum Master Skills m1 Slides PDFDocument66 pages1 Scrum Master Skills m1 Slides PDFSohaib Omer SalihNo ratings yet
- Classroom EtiquetteDocument1 pageClassroom EtiquetteJerrold MangaoNo ratings yet
- I Can Learn English 1Document100 pagesI Can Learn English 1IbetNo ratings yet
- PGCC 2022 HandbookDocument17 pagesPGCC 2022 HandbookhasnainNo ratings yet
- Wuolah Free Use of EnglishDocument20 pagesWuolah Free Use of EnglishCristina CórcolesNo ratings yet
- Proposal Project PrintingDocument13 pagesProposal Project PrintinglodewNo ratings yet
- Analogy - 10 Page - 01 PDFDocument10 pagesAnalogy - 10 Page - 01 PDFrifathasan13No ratings yet
- RNR150 Syllabus1Document14 pagesRNR150 Syllabus1Shaun SarvisNo ratings yet
- Golden Motor Cruise Controller User GuideDocument6 pagesGolden Motor Cruise Controller User Guidefrans_h_kNo ratings yet
- Public Health LawsDocument16 pagesPublic Health Lawscharlene bermasNo ratings yet
- Dr. Shikha Baskar: CHE154: Physical Chemistry For HonorsDocument22 pagesDr. Shikha Baskar: CHE154: Physical Chemistry For HonorsnishitsushantNo ratings yet
- Ccie DC Full Scale Labs PDFDocument185 pagesCcie DC Full Scale Labs PDFnaveedrana100% (2)
- Time Value of MoneyDocument11 pagesTime Value of MoneyRajesh PatilNo ratings yet
- Mathematics IDocument247 pagesMathematics IShreya PankajNo ratings yet
- DBMS - Lab CAT2 Code SWE1001 SLOT L47+48 DATE 16/10/19Document2 pagesDBMS - Lab CAT2 Code SWE1001 SLOT L47+48 DATE 16/10/19msroshi madhuNo ratings yet
- Penjelasan IMRAD StructureDocument2 pagesPenjelasan IMRAD Structureaji bondesNo ratings yet
- Peter Wink Resume Aug 2019Document1 pagePeter Wink Resume Aug 2019api-471317467No ratings yet
- HECKMAN, J. James - Schools, Skills and SynapsesDocument36 pagesHECKMAN, J. James - Schools, Skills and SynapsesAndré Gonçalves OliveiraNo ratings yet
- Economic GrowthDocument15 pagesEconomic GrowthANJULI AGARWALNo ratings yet
- As 4822-2008 External Field Joint Coatings For Steel PipelinesDocument8 pagesAs 4822-2008 External Field Joint Coatings For Steel PipelinesSAI Global - APAC0% (1)
- Billy Mitchel: The Fundamental Principles of Modern Monetary Economics (MMT/PDF)Document6 pagesBilly Mitchel: The Fundamental Principles of Modern Monetary Economics (MMT/PDF)mrwonkish100% (1)
- Media MetricsDocument28 pagesMedia MetricsPulkit ChhabraNo ratings yet
- Grammar Translation MethodDocument22 pagesGrammar Translation MethodCeyinNo ratings yet
- Database NotesDocument4 pagesDatabase NotesKanishka SeneviratneNo ratings yet
- "L8" Drive End: Operation - Assembly Instructions and Parts List ForDocument7 pages"L8" Drive End: Operation - Assembly Instructions and Parts List ForACCA PumpsNo ratings yet