Professional Documents
Culture Documents
Hydrocarbon Level I KVPY
Uploaded by
RAGHUL MOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Hydrocarbon Level I KVPY
Uploaded by
RAGHUL MCopyright:
Available Formats
Hydrocarbon Level :I KVPY
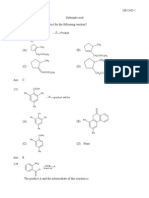
-----------------------------------------------------------------
1. The main product of dehydrohalogenation of 2-iodobutane is
(A) 1-butene (B) 2-butene
(C) n-butane (D) A and B equally
2. Anti Markownikov addition takes place in which of the following reactions
(A) CH3CH = CH2 + HBr 2
5 2 2 ( C H CO) O
(B) CH2 = CH –CH2Cl + HCl
Peroxide
(C) CH2 = CH - CH2OH + HCl
Peroxide
(D) CH2 = CH - CH2CH2CH2CH2OH + HCl
Peroxide
3. Which reacts fastest with conc. HCl
(A) (B)
CH CH OH 2 2 CH – CH3
OH
(C) CH3 (D) CH2 = CH –OH
CH3 – C – OH
CH3
4.
B
BH3 / THF
CH2 3
H O
A
H2O 2 / OH
A and B are
(A) (B)
both CH2OH both CH3
OH
(C) (D)
CH2OH, CH3 CH3 CH2OH,
OH OH
5.
CH2OH on dehydration with conc. H2SO4 forms predominantly:
(A) (B)
CH2 CH3
(C) (D) CH3
CH3
6. Which of the following will react with sodium metal?
(A) Ethene (B) Butyne-1
(C) Butyne-2 (D) Ethane
7. On being heated with alcoholic potassium hydroxide, neopentyl bromide gives mainly.
(A) 2-methyl-2-butene (B) 2-methyl-1-butene
(C) 2-butene (D) 2,2-dimethyl-1-butene
8. Treatment of a mixture of 1-chloropropane and 2-chloropropane with alcoholic KOH gives
(A) 1-propene (B) 2-propene
(C) isopropylene (D) none of these
9. The reaction HC CH CH2ClCHO takes place in
(A) HC CH
HOCl
(B) HC CH
HCl
peroxide
(C) HC CH
Cl2
(D) HC CH
Cl2
oxidizing agent
10. C3H8 + Cl2 C3H7Cl + HCl is an example of
light
(A) Substitution (B) Elimination
(C) Addition (D) Rearrangement reaction
11. How many types of carbon atoms are present in 2, 3, 3-trimethyl pentane?
(A) One (B) Two
(C) Three (D) Four
12. The order of stability of the alkenes
R2C CR2 , R2C CHR, R2C CH2 , RCH CHR, RCH CH2 is
(I) (II ) (III ) (IV ) (V)
(A) I II III IV V (B) I = II III IV V
(C) II I IV III V (D) V IV III I II
13. 2-butyne + NaNH2 X + NH3, X will be
Paraf f in
(A) sodium salt of 2-butyne (B) sodium salt of 1-butyne
(C) 2-butyne (D) 1-butyne
14. Which of the following has least restricted rotation around carbon carbon bond?
(A) Ethane (B) Ethylene
(C) Acetylene (D) Hexachlorohexane
15. A mixture of ethyl bromide and n-propylbromide is subjected to Wurtz reaction. The
hydrocarbon that will not be formed is
(A) n-butane (B) n-propane
(C) n-pentane (D) n-hexane
16. Natural gas is composed primarily of
(A) Methane (B) n-butane
(C) n-octane (D) a mixture of octanes
17. The highest boiling point is expected for
(A) Isooctane (B) n-octane
(C) 2, 2, 3, 3-tetramethylbutane (D) n-butane
18. The total number of structural isomers possible for structure C3H6 are
(A) 1 (B) 2
(C) 3 (D) 4
19. Anti Markownikoffs addition of HBr is not observed in
(A) Propene (B) Butene
(C) Butene-2 (D) Pentene-2
20. Propene, CH3CH=CH2 can be converted into 1-proponol by oxidation. Which set of
reagents among the following is ideal to effect the conversion
(A) alkaline KMnO4 (B) B2H6 & alk H2O2
(C) O3 (Zn Dust) (D) O2O4/CHCl3
You might also like
- Practice TestDocument14 pagesPractice TestHimanshu JindalNo ratings yet
- Alcohols IIT PACEDocument19 pagesAlcohols IIT PACEDushyant GuptaNo ratings yet
- Alkanes - Alkenes - Alkynes - DPP 2Document3 pagesAlkanes - Alkenes - Alkynes - DPP 2Vishal_93No ratings yet
- A2 PDFDocument3 pagesA2 PDFJatin SinglaNo ratings yet
- Organic 6 CDocument26 pagesOrganic 6 CDr.Rajarshi PatelNo ratings yet
- Chem 212 Alkyl Halide Problems 3Document1 pageChem 212 Alkyl Halide Problems 3kevinamyNo ratings yet
- Chem 212 Alkyl Halide Problems 4Document1 pageChem 212 Alkyl Halide Problems 4kevinamyNo ratings yet
- 13FINALSHEET06STEREOISOMERDocument23 pages13FINALSHEET06STEREOISOMERarryan keshanNo ratings yet
- Chemistry 108M Final Exam Practice 1Document8 pagesChemistry 108M Final Exam Practice 1Norma Leticia RamosNo ratings yet
- Prince Singh: Physical & Inorganic ChemistryDocument5 pagesPrince Singh: Physical & Inorganic ChemistryJatin SinglaNo ratings yet
- PACE Final Lap (Organic Chemistry) PDFDocument152 pagesPACE Final Lap (Organic Chemistry) PDFAman AdatiaNo ratings yet
- ADV. I 57 - 64 (Exercise 3)Document8 pagesADV. I 57 - 64 (Exercise 3)Aditya ShahNo ratings yet
- Part - I: Objective Questions: Section A: Geometrical IsomerismDocument10 pagesPart - I: Objective Questions: Section A: Geometrical IsomerismTejas pawarNo ratings yet
- TEST 2 GOC & POC Tough by S.K.sinha See Chemistry Animations atDocument3 pagesTEST 2 GOC & POC Tough by S.K.sinha See Chemistry Animations atmyiitchemistryNo ratings yet
- Alcohols & EtherDocument18 pagesAlcohols & EtherRaju SinghNo ratings yet
- Liquid SolutionsDocument9 pagesLiquid SolutionsrockNo ratings yet
- Chem 212 Alkyl Halide Problems 2Document1 pageChem 212 Alkyl Halide Problems 2kevinamy100% (1)
- Solved Example: Chemistry For Neet & AiimsDocument24 pagesSolved Example: Chemistry For Neet & AiimsAnup KNo ratings yet
- IIT JEE Chemistry Revision on Liquid Solutions and Colligative PropertiesDocument5 pagesIIT JEE Chemistry Revision on Liquid Solutions and Colligative PropertiesJatin Singla100% (1)
- General Organic Chemistry - Sheet - 10 - 11 & 12) (Hyperconjugation & Aromaticity) Level - 1 1Document10 pagesGeneral Organic Chemistry - Sheet - 10 - 11 & 12) (Hyperconjugation & Aromaticity) Level - 1 1IznnxxkozsksnndNo ratings yet
- Caieee04fisica PDFDocument15 pagesCaieee04fisica PDFRafaelNo ratings yet
- Solution - Colligative Properties Solutions PDFDocument25 pagesSolution - Colligative Properties Solutions PDFGOURISH AGRAWALNo ratings yet
- Assignment 1Document3 pagesAssignment 1Andrew_Wong_8492No ratings yet
- Carbonyl Compound WorksheetDocument25 pagesCarbonyl Compound WorksheetOmendra SinghNo ratings yet
- Dipole Moments in Organic CHEMISTRYDocument18 pagesDipole Moments in Organic CHEMISTRYBalraj Dhillon100% (2)
- Mole Concept-2: Oxidation, Reduction, and Balancing Redox EquationsDocument38 pagesMole Concept-2: Oxidation, Reduction, and Balancing Redox EquationsR S.NagiNo ratings yet
- Maths QuesDocument2 pagesMaths QuesKunalSinghNo ratings yet
- (02-12-14) AlkenesDocument4 pages(02-12-14) Alkenessasi.curieNo ratings yet
- JEEMain S-Block QuestionsDocument7 pagesJEEMain S-Block QuestionsSnehaNo ratings yet
- 03ElectronicdisplacementEffects Exercise Send1Document33 pages03ElectronicdisplacementEffects Exercise Send1Aaryan Keshan100% (1)
- Exercise-01 Check Your Grasp: K Cro Dil. HCLDocument20 pagesExercise-01 Check Your Grasp: K Cro Dil. HCLAkashGauravNo ratings yet
- G R Reduction AlkaneDocument43 pagesG R Reduction AlkaneManthan HaritashNo ratings yet
- GRiGNARD REAGENT!!Document22 pagesGRiGNARD REAGENT!!GazalNo ratings yet
- CH CH CHCH CH H CH CH CH CH CH CH H CH: Byvineet Khatri SirDocument13 pagesCH CH CHCH CH H CH CH CH CH CH CH H CH: Byvineet Khatri Sirsarvesh goyalNo ratings yet
- Reaction IntermediatesDocument32 pagesReaction Intermediatestechno studioNo ratings yet
- Reduction, Oxidation - Hydrolysis Exercise PDFDocument24 pagesReduction, Oxidation - Hydrolysis Exercise PDFGOURISH AGRAWAL100% (3)
- Carbocation RearrangementDocument4 pagesCarbocation RearrangementManas J. AggarwalNo ratings yet
- 12th Chem Exemplar PDFDocument288 pages12th Chem Exemplar PDFRalston King Stulla ChambersNo ratings yet
- Chemical Kinetics (M) PDFDocument41 pagesChemical Kinetics (M) PDFNalla Umapathi Reddy75% (4)
- National Standard Examination in Chemistry 2012-2013: Association of Chemistry TeachersDocument15 pagesNational Standard Examination in Chemistry 2012-2013: Association of Chemistry TeachersChinmaya SinghNo ratings yet
- Alkenes Alkynes Oxidation PDFDocument32 pagesAlkenes Alkynes Oxidation PDFRamuNo ratings yet
- TARGET IIT-JEE HYDROCARBONS REACTION PRACTICEDocument31 pagesTARGET IIT-JEE HYDROCARBONS REACTION PRACTICEHarsh VardhanNo ratings yet
- Solubility Product ProblemsDocument4 pagesSolubility Product ProblemsT sidharth100% (1)
- ORGANIC CHEMISTRY DPPDocument7 pagesORGANIC CHEMISTRY DPPAshish RanjanNo ratings yet
- Bansal Classes Organic Chemistry Study Material For IIT JEEDocument477 pagesBansal Classes Organic Chemistry Study Material For IIT JEEAditya Kavuluri40% (5)
- Stereoisomerism VKP SirDocument49 pagesStereoisomerism VKP SirSandeep ReddyNo ratings yet
- Aldol Reaction - ChemistryDocument7 pagesAldol Reaction - ChemistryGamer HelperNo ratings yet
- Goc Question Bank: Complete Course On Organic Chemistry For JEE 2020Document8 pagesGoc Question Bank: Complete Course On Organic Chemistry For JEE 2020Vishvas Ranjan SrivastavaNo ratings yet
- 5 DPP - 56to81 - FinalDocument44 pages5 DPP - 56to81 - FinalArnab KumarNo ratings yet
- T1-1 TDocument30 pagesT1-1 TFRENCHONLYNo ratings yet
- Energy AnsDocument3 pagesEnergy AnskevinamyNo ratings yet
- Class XII Organic Chemistry questionsDocument4 pagesClass XII Organic Chemistry questionsSelcouth elysianNo ratings yet
- Alkyl HalidesDocument20 pagesAlkyl HalidesShivam Gupta0% (1)
- OC - Halogen Derivatives - E - CE PDFDocument42 pagesOC - Halogen Derivatives - E - CE PDFAbhinesh SinghNo ratings yet
- Reactions of Alkenes: CC HX C HX C Markovnikov's OrientationDocument8 pagesReactions of Alkenes: CC HX C HX C Markovnikov's OrientationMarc RitzNo ratings yet
- Goc & Eas Test-IiDocument7 pagesGoc & Eas Test-IiAniket GuptaNo ratings yet
- CARBOXYLIC ACID REACTIONSDocument24 pagesCARBOXYLIC ACID REACTIONSGulshan kumarNo ratings yet
- Sheet 1Document11 pagesSheet 1Zooper lNo ratings yet
- GujCET - D26 Mar 2023Document34 pagesGujCET - D26 Mar 2023aadityabhagchandaniNo ratings yet
- Isomerism DPPDocument4 pagesIsomerism DPPRAGHUL MNo ratings yet
- Dictionary of QuranDocument22 pagesDictionary of QuranSyed HarrisNo ratings yet
- Disease Test ReviewDocument2 pagesDisease Test ReviewRAGHUL MNo ratings yet
- Hydrocarbon Level I KVPYDocument2 pagesHydrocarbon Level I KVPYRAGHUL MNo ratings yet
- Topics From Botany: Kvpy Analysis: Sa StreamDocument12 pagesTopics From Botany: Kvpy Analysis: Sa StreamAnonymous EyhxoejNo ratings yet
- Block A Collides With Block B. After The Collisions The Two Blocks Stick Together. Which of The Following Is True?Document2 pagesBlock A Collides With Block B. After The Collisions The Two Blocks Stick Together. Which of The Following Is True?JoØrsh Ênrique Tu Xikytø NînîØflowNo ratings yet
- Twenty Frequently Asked Questions About Mit OpencoursewareDocument5 pagesTwenty Frequently Asked Questions About Mit OpencoursewareRAGHUL MNo ratings yet
- Vci Neet PN 2019Document1 pageVci Neet PN 2019RAGHUL MNo ratings yet
- 3 Trigonometric FunctionsDocument11 pages3 Trigonometric FunctionsSatyam PandeyNo ratings yet
- TNJFU UG Admission Prospectus 2018 19 TN CandidatesDocument39 pagesTNJFU UG Admission Prospectus 2018 19 TN CandidatesRAGHUL MNo ratings yet
- Tamil Nadu Dr. J. Jayalalithaa Fisheries University: 1 Counselling For Ug Admission 2019-2020Document1 pageTamil Nadu Dr. J. Jayalalithaa Fisheries University: 1 Counselling For Ug Admission 2019-2020RAGHUL MNo ratings yet
- U Greg AlliedDocument4 pagesU Greg AlliedgdmanNo ratings yet
- Worksheet On KVPY: Fiitjee BengaluruDocument3 pagesWorksheet On KVPY: Fiitjee BengaluruRAGHUL MNo ratings yet
- Fee Notification 1583Document1 pageFee Notification 1583RAGHUL MNo ratings yet
- Calculus, Differential Equations and Physics Course OverviewDocument73 pagesCalculus, Differential Equations and Physics Course OverviewRAGHUL MNo ratings yet
- Online Couseling ProcedureDocument3 pagesOnline Couseling ProcedureRAGHUL MNo ratings yet
- Central University of Tamil Nadu, Thiruvarur PROVISIONAL SELECTION LIST - Integrated M.Sc. (Chemistry)Document1 pageCentral University of Tamil Nadu, Thiruvarur PROVISIONAL SELECTION LIST - Integrated M.Sc. (Chemistry)RAGHUL MNo ratings yet
- NAHEP Invites Concept Notes for 3rd Round FundingDocument12 pagesNAHEP Invites Concept Notes for 3rd Round FundingRAGHUL MNo ratings yet
- Advertisement English HindiDocument2 pagesAdvertisement English HindiRAGHUL MNo ratings yet
- MBA in Fisheries Management at TNJFU Business SchoolDocument13 pagesMBA in Fisheries Management at TNJFU Business SchoolRAGHUL MNo ratings yet
- Tamilnadu Veterinary and Animal Sciences University Undergraduate Admission 2019 - 2020Document1 pageTamilnadu Veterinary and Animal Sciences University Undergraduate Admission 2019 - 2020RAGHUL MNo ratings yet
- Quantitative Techniques in Textile EngineeringDocument26 pagesQuantitative Techniques in Textile EngineeringRAGHUL MNo ratings yet
- Solutions To BookDocument48 pagesSolutions To BookRAGHUL MNo ratings yet
- TNJFU UG Admission 2019Document44 pagesTNJFU UG Admission 2019RAGHUL MNo ratings yet
- Vacant / Left Over Seat Matrix After Mop Up / Final Round (AIEEA UG 2019)Document9 pagesVacant / Left Over Seat Matrix After Mop Up / Final Round (AIEEA UG 2019)RAGHUL MNo ratings yet
- TNJFU UG Admission 2019Document44 pagesTNJFU UG Admission 2019RAGHUL MNo ratings yet
- Prospectus Diploma 2019 20Document7 pagesProspectus Diploma 2019 20RAGHUL MNo ratings yet
- GRB Physics For Competitions Vol 1Document853 pagesGRB Physics For Competitions Vol 1Shubham Gupta85% (20)
- 1 Mechanics: Cbse Neet JEE Mains AdvancedDocument11 pages1 Mechanics: Cbse Neet JEE Mains Advancedvsrajkumar100% (6)
- 1 Au NPs Thin Films Fabricated by Electrophoretic Deposition Method For Highly Sensitive SERS Application Odi YesDocument7 pages1 Au NPs Thin Films Fabricated by Electrophoretic Deposition Method For Highly Sensitive SERS Application Odi Yesben0706No ratings yet
- Turro NSFHighlight IndustrialDocument5 pagesTurro NSFHighlight IndustrialDrShashikant DargarNo ratings yet
- ICAMM-2016 Conference Technical Sessions ScheduleDocument19 pagesICAMM-2016 Conference Technical Sessions SchedulePurna Suresh PedamalluNo ratings yet
- Materials Chemistry and Physics: Y.Y. Song, X.Y. Li, L.J. Rong, Y.Y. Li, T. NagaiDocument7 pagesMaterials Chemistry and Physics: Y.Y. Song, X.Y. Li, L.J. Rong, Y.Y. Li, T. NagaiEdward Giovanni Rodriguez AriasNo ratings yet
- Business Grammar Builder Intermediate To Upper Int - 5ada31c07f8b9ad4148b45aaDocument14 pagesBusiness Grammar Builder Intermediate To Upper Int - 5ada31c07f8b9ad4148b45aaMarko MaticNo ratings yet
- Design Pipe Systems Pumps Gea Hilge Manual 272424Document60 pagesDesign Pipe Systems Pumps Gea Hilge Manual 272424Geferson GonçalvesNo ratings yet
- Amino + Nitro Compounds Class XII NotesDocument62 pagesAmino + Nitro Compounds Class XII NotesAditya BhattNo ratings yet
- Flammability of A Gas MixtureDocument6 pagesFlammability of A Gas Mixturebldp03No ratings yet
- Chem Sem 1 Q &A PDFDocument9 pagesChem Sem 1 Q &A PDFevacuate clashNo ratings yet
- Nano/Microparticles For Retina and Posterior Diseases: Anita Patel, Jayvadan K. Patel, and Elie Beit-YannaiDocument24 pagesNano/Microparticles For Retina and Posterior Diseases: Anita Patel, Jayvadan K. Patel, and Elie Beit-YannaiFer RodriguezNo ratings yet
- E747 97 Wire IQI PDFDocument14 pagesE747 97 Wire IQI PDFAmith100% (1)
- NLAM - National Library of Ayurved MedicineDocument2 pagesNLAM - National Library of Ayurved MedicineJack LeeNo ratings yet
- CadDocument8 pagesCadRamesh Babu GarlapatiNo ratings yet
- ASTM A262, CorrosietestenDocument2 pagesASTM A262, CorrosietestenSales HBS Solutions100% (1)
- Nov PistonsDocument2 pagesNov PistonsRICARDO REYESNo ratings yet
- 9501-PP-QA-009 Piping Leak Test Procedure R-0Document27 pages9501-PP-QA-009 Piping Leak Test Procedure R-0Josh RuddyNo ratings yet
- Bends Requirement As Ibr & AsmeDocument3 pagesBends Requirement As Ibr & AsmeSimbu ArasanNo ratings yet
- Periodic TableDocument1 pagePeriodic TableChemist MookaNo ratings yet
- Agma 926-C99Document16 pagesAgma 926-C99Mehul Bansal100% (2)
- Conductive Polymers: Properties, Synthesis and ApplicationsDocument7 pagesConductive Polymers: Properties, Synthesis and ApplicationsDHANUSH RacerNo ratings yet
- GC Analysis Derivatization ReactionsDocument28 pagesGC Analysis Derivatization Reactionsfarkad rawiNo ratings yet
- Methods For Chemical Analysis of Steels: Indian StandardDocument5 pagesMethods For Chemical Analysis of Steels: Indian StandardGopalMahantaNo ratings yet
- Steel Castings, Carbon, For General ApplicationDocument4 pagesSteel Castings, Carbon, For General ApplicationVIKAS DAHIYA100% (1)
- Mothballing Requires More Than Idle ThoughtDocument4 pagesMothballing Requires More Than Idle Thoughtfawmer61No ratings yet
- NPTEL Course List Jan 2022Document24 pagesNPTEL Course List Jan 2022PathanSameerKhanNo ratings yet
- EllinghamDocument19 pagesEllinghamJuan Ignacio GonzálezNo ratings yet
- Homework #5. Control Volume Analysis Using Energy.: Universidad de Guanajuato, DICIS. TermodinámicaDocument3 pagesHomework #5. Control Volume Analysis Using Energy.: Universidad de Guanajuato, DICIS. TermodinámicaTravis BickleNo ratings yet
- Guide To Classification and Wall Chart - 130208Document2 pagesGuide To Classification and Wall Chart - 130208Farid AmarullahNo ratings yet
- Modeling Biologics, Antibodies & ProteinsDocument2 pagesModeling Biologics, Antibodies & Proteinsthamizh555No ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)
- Piping and Pipeline Calculations Manual: Construction, Design Fabrication and ExaminationFrom EverandPiping and Pipeline Calculations Manual: Construction, Design Fabrication and ExaminationRating: 4 out of 5 stars4/5 (18)
- Functional Safety from Scratch: A Practical Guide to Process Industry ApplicationsFrom EverandFunctional Safety from Scratch: A Practical Guide to Process Industry ApplicationsNo ratings yet
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Nuclear Energy in the 21st Century: World Nuclear University PressFrom EverandNuclear Energy in the 21st Century: World Nuclear University PressRating: 4.5 out of 5 stars4.5/5 (3)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- Coating and Drying Defects: Troubleshooting Operating ProblemsFrom EverandCoating and Drying Defects: Troubleshooting Operating ProblemsRating: 5 out of 5 stars5/5 (1)
- Trevor Kletz Compendium: His Process Safety Wisdom Updated for a New GenerationFrom EverandTrevor Kletz Compendium: His Process Safety Wisdom Updated for a New GenerationNo ratings yet
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- An Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksFrom EverandAn Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksRating: 5 out of 5 stars5/5 (1)
- Chemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastFrom EverandChemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastNo ratings yet
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- Gas-Liquid And Liquid-Liquid SeparatorsFrom EverandGas-Liquid And Liquid-Liquid SeparatorsRating: 3.5 out of 5 stars3.5/5 (3)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)