Professional Documents
Culture Documents
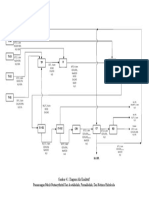
Design of a 23,000 Ton/Year Hexamine Plant from Formaldehyde and Ammonia
Uploaded by
fadliOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Design of a 23,000 Ton/Year Hexamine Plant from Formaldehyde and Ammonia
Uploaded by
fadliCopyright:
Available Formats
T = 30 0C
T = -33 0C PRARANCANGAN PABRIK HEXAMINE DARI
P = 1 atm
P = 1 atm
NH3 (l) = 99,5%
CH2O (l) = 37% FORMALDEHID DAN AMONIA DENGAN PROSES
CH3OH (l) = 2,5%
H2O (l) = 0,5% LEONARD DENGAN KAPASITAS 23.000 TON /TAHUN
H2O (l) = 60,5%
X = 85% T = 40 0C
Reaktor 1 CH2O + NH3 C6H12N4 + H2O
P = 16 atm
C6H12N4 (l)
H2O (l)
CH3OH (l)
CH2O (l)
NH3 (l)
T = 40 0C CH2O + NH3 C6H12N4 + H2O
X = 15% Reaktor 2 P = 16 atm
T = 30 0C T = 80 0C
C6H12N4 (l) T = 86 0C H2O (l) H2O (g)
H2O (l) T = 54 0C H2O (g) CH3OH (l) CH3OH (g)
CH3OH (l) H2O (g) CH3OH (g) C6H12N4 (l)
CH2O (l) CH3OH (g) Suhu Udara Masuk (oC) 130
NH3 (l) CH2O (g) Suhu Udara Keluar (oC) 62
NH3 (g)
Rotary
Evaporator 1 Evaporator 2 Centrifuge Silo
Dryer
T = 80 0C
P = 1 atm
T = 54 0C H2O (l) T = 86 0C H2O (l) T = 30 0C H2O (l) Produk
P = 0,31 atm CH3OH (l) P = 0,28 atm CH3OH (l) P = 1 atm CH3OH (l) T = 300C
atm C6H12N4 (l) atm C6H12N4 (l) C6H12N4 (l) P = 1 atm
Titik Didih (oC) C6H12N4 (s) C6H12N4 (s) C6H12N4 (s) H2O (l) = 0,095%
H2O 100 CH3OH (l) = 0,05%
C6H12N4 (l) = 1,9%
CH3OH 64,7
C6H12N4 (s) = 98%
CH2O 98
NH3 -33
You might also like
- Antiseptic and Disinfectant-KDBDocument43 pagesAntiseptic and Disinfectant-KDBKiran100% (3)
- Chapter 5 HydrocarbonDocument25 pagesChapter 5 Hydrocarbonmeshal retteryNo ratings yet
- Proper Cleaning Solutions FinalDocument4 pagesProper Cleaning Solutions Finalapi-555520296No ratings yet
- Stoichiometry Calculations With Chemical FormulasDocument55 pagesStoichiometry Calculations With Chemical FormulassaneleNo ratings yet
- Gas Pipeline Hydraulic Analysis CalculationDocument10 pagesGas Pipeline Hydraulic Analysis Calculationbalakrishna100% (3)
- Cooling & Heating Division: Steam DrivenDocument16 pagesCooling & Heating Division: Steam DrivenMontu MiaNo ratings yet
- 17 Petrucci10e CSMDocument104 pages17 Petrucci10e CSMElah PalaganasNo ratings yet
- Mandi DeepDocument14 pagesMandi DeepRohit ThannaNo ratings yet
- Registered Topical Antiseptics and Antibacterials ListDocument15 pagesRegistered Topical Antiseptics and Antibacterials ListSerjoe N. RosalNo ratings yet
- Diagram Alir Pabrik Urea FormaldehidDocument2 pagesDiagram Alir Pabrik Urea Formaldehid23 MispaNo ratings yet
- CH3OH Pengolahan Limbah Ke UnitDocument2 pagesCH3OH Pengolahan Limbah Ke UnitMarsya MasyitaNo ratings yet
- DoE Politecnico di Milano WGS Reactor Outlet TemperatureDocument6 pagesDoE Politecnico di Milano WGS Reactor Outlet TemperatureAlejandro BarreraNo ratings yet
- ChE 111P: Heat and mass balances of humidification and condensation processesDocument6 pagesChE 111P: Heat and mass balances of humidification and condensation processesMateo PremarionNo ratings yet
- Material Balance CalculationsDocument10 pagesMaterial Balance CalculationsAnjali BalmikiNo ratings yet
- Diagram Alir Kualitatif FixDocument1 pageDiagram Alir Kualitatif FixRifki AzharNo ratings yet
- Exercise 3 Outlet Temperature of A WGS ReactorDocument6 pagesExercise 3 Outlet Temperature of A WGS ReactorMiguelCardonaSalazarNo ratings yet
- Approximate 1H and 13C NMR ShiftsDocument5 pagesApproximate 1H and 13C NMR ShiftsCamilo AndresNo ratings yet
- Psychrometric Charts (Humidity Chart)Document7 pagesPsychrometric Charts (Humidity Chart)ccami709No ratings yet
- CHE 102 Package - Final 2010 PDFDocument29 pagesCHE 102 Package - Final 2010 PDFzain-hiraniNo ratings yet
- Thermodynamics 2Document5 pagesThermodynamics 2Charlotte HooperNo ratings yet
- Exercise 4 Outlet Temperature and Composition of A WGS ReactorDocument7 pagesExercise 4 Outlet Temperature and Composition of A WGS ReactorMiguelCardonaSalazarNo ratings yet
- Drying Tower & Sulphur BurnerDocument18 pagesDrying Tower & Sulphur BurnerAhmed Qutb AkmalNo ratings yet
- Suzuki ReactionDocument2 pagesSuzuki ReactionKIRAN ALLUNo ratings yet
- Penentuan Kapasitas Panas Kalorimeter: Analisis DataDocument5 pagesPenentuan Kapasitas Panas Kalorimeter: Analisis DataNovita RahmawatiNo ratings yet
- Chapter 4: Energy Balance: I Ki PiDocument13 pagesChapter 4: Energy Balance: I Ki PiAhmed Qutb AkmalNo ratings yet
- Test-Ii Chemistry: Part-I Section-I Single Correct Choice Type 1. (D)Document19 pagesTest-Ii Chemistry: Part-I Section-I Single Correct Choice Type 1. (D)pro7No ratings yet
- Second Methode + ExercisesDocument8 pagesSecond Methode + ExercisesZeina Abi FarrajNo ratings yet
- 9.diagram Alir Kuantitatif 4xDocument2 pages9.diagram Alir Kuantitatif 4xRafi Theda PrabawaNo ratings yet
- 4.4.2 Energy Balance For Sulphur BurnerDocument5 pages4.4.2 Energy Balance For Sulphur BurnerAhmed Qutb AkmalNo ratings yet
- Informe 8Document4 pagesInforme 8Miguel MorenoNo ratings yet
- ChE102 Final Exam ReviewDocument29 pagesChE102 Final Exam ReviewalyNo ratings yet
- Grafik Penentuan KalorDocument6 pagesGrafik Penentuan KalorRolan 06No ratings yet
- Quaternary Ammonium Salts PDFDocument11 pagesQuaternary Ammonium Salts PDFBridgett Lanette RobinsonNo ratings yet
- Cálculos Químicos: Cálculos Del Experimento 1: "Volumen Molar de Un Gas"Document4 pagesCálculos Químicos: Cálculos Del Experimento 1: "Volumen Molar de Un Gas"Carl AcuñaNo ratings yet
- Applications 1st ODE PDFDocument12 pagesApplications 1st ODE PDFMahmoud MahmoudNo ratings yet
- 100S120 CS19L01Document38 pages100S120 CS19L01b101112154No ratings yet
- Lampiran II YaaaDocument28 pagesLampiran II YaaaArananda Dwi PutriNo ratings yet
- P2 - Chp5 - Titrimetric AnalysisDocument77 pagesP2 - Chp5 - Titrimetric AnalysisNguyễn Hoàng QuânNo ratings yet
- Solving Chemical Reaction Problems Using Extent of Reaction and Atomic Balance MethodsDocument4 pagesSolving Chemical Reaction Problems Using Extent of Reaction and Atomic Balance MethodsKyle SaylonNo ratings yet
- Q2 ch125P RetakeDocument5 pagesQ2 ch125P RetakeFlorenceNo ratings yet
- Chemical Reaction Engineering Lab: Experiment No. 02Document4 pagesChemical Reaction Engineering Lab: Experiment No. 02Varun Sharma100% (1)
- Solution for IUPAC names and reaction productsDocument4 pagesSolution for IUPAC names and reaction productsPahal Kumari SinhaNo ratings yet
- CHEMICAL REACTION KINETICS LABDocument54 pagesCHEMICAL REACTION KINETICS LABahmad RaoNo ratings yet
- BBBBBBBBBBBBBXDocument43 pagesBBBBBBBBBBBBBXTri YaniNo ratings yet
- Ef0c00890 Si 001Document6 pagesEf0c00890 Si 001Austin SmithNo ratings yet
- Ejercicios PsicrometríaDocument10 pagesEjercicios Psicrometríamaria paulaNo ratings yet
- CHM131 - Chapter 5 - The Gases StateDocument54 pagesCHM131 - Chapter 5 - The Gases StateLeo PietroNo ratings yet
- Q2 ch125P 1st QDocument7 pagesQ2 ch125P 1st QFlorenceNo ratings yet
- Corriges Des Exercices (Initiation)Document2 pagesCorriges Des Exercices (Initiation)عادل الحمديNo ratings yet
- Topic 05 Chemical Equilibrium Tutorial PDFDocument21 pagesTopic 05 Chemical Equilibrium Tutorial PDFTimNo ratings yet
- CEQUENCE 1 f 2Document6 pagesCEQUENCE 1 f 2Abderrahim ZaboujNo ratings yet
- 2011 Stoichiometry Test MEMODocument4 pages2011 Stoichiometry Test MEMOJackson MakgolengNo ratings yet
- TutorialDocument27 pagesTutorialSiti NuraqidahNo ratings yet
- Determine Diffusion Coefficient of Acetone VapourDocument13 pagesDetermine Diffusion Coefficient of Acetone VapourerickhadinataNo ratings yet
- Problems: Syn To The Methyl GroupDocument1 pageProblems: Syn To The Methyl Grouppanda biruNo ratings yet
- Excel Psych Functions HELP FileDocument39 pagesExcel Psych Functions HELP FilemshahNo ratings yet
- CP DT: Gas Butano (Entrada)Document3 pagesCP DT: Gas Butano (Entrada)Diego TavizónNo ratings yet
- Exercise 6Document3 pagesExercise 6jay TanshiNo ratings yet
- PH O P Total P H O: A) Molal SaturationDocument5 pagesPH O P Total P H O: A) Molal SaturationMiraNo ratings yet
- Atk FixDocument9 pagesAtk FixRama SlaluhappyNo ratings yet
- Ans SW Recycle PurgeDocument3 pagesAns SW Recycle PurgeFlorenceNo ratings yet
- ANNISSA CALCULATES ADIABATIC FLAME TEMPERATUREDocument13 pagesANNISSA CALCULATES ADIABATIC FLAME TEMPERATUREAnnissa RiskyNo ratings yet
- Matriculation Chemistry (Amino Acids) Part 2Document10 pagesMatriculation Chemistry (Amino Acids) Part 2ridwanNo ratings yet
- Unifac GroupsDocument10 pagesUnifac GroupsSuryaprakash DigavalliNo ratings yet
- Solution - Practice Paper 3Document12 pagesSolution - Practice Paper 3Jitendra UdawantNo ratings yet
- Critical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsFrom EverandCritical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsNo ratings yet
- LT15595 SelfCleaningPrecleaner GBDocument4 pagesLT15595 SelfCleaningPrecleaner GBfadliNo ratings yet
- 0.05m 0.045m 0.04m 0.035m 0.03m 0.04m: 0.15m 0.15m 0.20m 0.23m 0.08mDocument4 pages0.05m 0.045m 0.04m 0.035m 0.03m 0.04m: 0.15m 0.15m 0.20m 0.23m 0.08mfadliNo ratings yet
- LT15595 SelfCleaningPrecleaner GBDocument4 pagesLT15595 SelfCleaningPrecleaner GBfadliNo ratings yet
- Sari Dumai SejatiDocument9 pagesSari Dumai SejatifadliNo ratings yet
- Fruit Set and Oil Palm Bunch ComponentsDocument11 pagesFruit Set and Oil Palm Bunch ComponentsIkrar Nusantara PutraNo ratings yet
- TRANE Steam Driven Absorption ChillersDocument41 pagesTRANE Steam Driven Absorption ChillersMarian Vančo100% (1)
- Eligible Masters Programmes Sisgp and Sissa 2021 - 2022 PDFDocument66 pagesEligible Masters Programmes Sisgp and Sissa 2021 - 2022 PDFLearnmoreNo ratings yet
- Heat Exchanger GuideDocument21 pagesHeat Exchanger GuideVipin PandeyNo ratings yet
- Brochure-Filter Press.-TORODocument12 pagesBrochure-Filter Press.-TOROSebastian Gomez BetancourtNo ratings yet
- ADBflyer 2014Document2 pagesADBflyer 2014fadliNo ratings yet
- Curriculum Vitae ExampleDocument2 pagesCurriculum Vitae ExamplefadliNo ratings yet
- Asepsis 11-07-2023Document3 pagesAsepsis 11-07-2023sawantheloveguruNo ratings yet
- Disinectant Label BAC 50%Document1 pageDisinectant Label BAC 50%alvNo ratings yet
- Guidelines: Interim List of Household Products and Active Ingredients For Disinfection of The COVID-19 VirusDocument13 pagesGuidelines: Interim List of Household Products and Active Ingredients For Disinfection of The COVID-19 VirusRodolfoANo ratings yet
- Biochek Scrub: Chlorhexidine Gluconate Solution Antiseptic Cleansing SolutionDocument1 pageBiochek Scrub: Chlorhexidine Gluconate Solution Antiseptic Cleansing SolutionSamba Siva RaoNo ratings yet
- Price List E-Katalog 20Document1 pagePrice List E-Katalog 20Kuncoro Ambra ZNo ratings yet
- CBC COVID19 Product List 3272020 PDFDocument16 pagesCBC COVID19 Product List 3272020 PDFFarrukh KhanNo ratings yet
- Novel Coronavirus Fighting Products ListDocument6 pagesNovel Coronavirus Fighting Products ListActionNewsJaxNo ratings yet
- Antiseptic SolutionsDocument1 pageAntiseptic SolutionsesteriplasNo ratings yet
- Critical solution temperature of phenol-water systemDocument6 pagesCritical solution temperature of phenol-water systemFaheeraNo ratings yet
- Fișa de Lucru Nr. 2 Sarcina de Lucru: Denumiți Următorii AlcooliDocument2 pagesFișa de Lucru Nr. 2 Sarcina de Lucru: Denumiți Următorii Alcoolitp VladNo ratings yet
- Antiseptik KulitDocument27 pagesAntiseptik KulitAzrul AzlanNo ratings yet
- Guidelines for household disinfection of COVID-19Document8 pagesGuidelines for household disinfection of COVID-19atllNo ratings yet
- E200-E300 Preservatives: Number Name FunctionDocument3 pagesE200-E300 Preservatives: Number Name FunctionAlicia Tan Suat HongNo ratings yet
- 337-1-2015 حمض السوربيك وأملاحه المستخدمة فى حفظ المنتجات الغذائية الجزء الأول - حمض السوربيكDocument10 pages337-1-2015 حمض السوربيك وأملاحه المستخدمة فى حفظ المنتجات الغذائية الجزء الأول - حمض السوربيكDrafaf MahmoudNo ratings yet
- Tindak Lanjut Hasil Inspeksi Ketersediaan Dan Pelabelanbahan Berbahaya Dan Beracun (B3) Rsud Talisayan BulanDocument6 pagesTindak Lanjut Hasil Inspeksi Ketersediaan Dan Pelabelanbahan Berbahaya Dan Beracun (B3) Rsud Talisayan BulanHady Waza YaaNo ratings yet
- Koefisien FenolDocument28 pagesKoefisien FenolWirda WagiantiNo ratings yet
- Dubai Municipality Lists Approved B2B BiocidesDocument14 pagesDubai Municipality Lists Approved B2B BiocidesAhmed FathyNo ratings yet
- DescoseptAF - EN - 0515 For Medical Device and Surface Clean PDFDocument3 pagesDescoseptAF - EN - 0515 For Medical Device and Surface Clean PDFPooja TiwariNo ratings yet
- Updated PPT Surgical Hand WashingDocument13 pagesUpdated PPT Surgical Hand WashingInsatiable CleeNo ratings yet
- Iodium Pada Telur AsinDocument6 pagesIodium Pada Telur AsinBayu WaeNo ratings yet
- Assign Chem in EverydayLife 16Document1 pageAssign Chem in EverydayLife 16Shubham AsthanaNo ratings yet
- SESSION 2021-22: Chemistry Investigatory ProjectDocument14 pagesSESSION 2021-22: Chemistry Investigatory ProjectsaharyaNo ratings yet
- CBC COVID19 Product List 3 - 25 - 2020 PDFDocument15 pagesCBC COVID19 Product List 3 - 25 - 2020 PDFIgnaMarounNo ratings yet
- LysolDocument4 pagesLysolDiego Ferney GuarinNo ratings yet
- ساختار لوئیسDocument2 pagesساختار لوئیسapi-3706290100% (1)
- Inglés English - Activity - The Big Bang Theory - Sheldon The Germaphobe ANSWER KEYSDocument3 pagesInglés English - Activity - The Big Bang Theory - Sheldon The Germaphobe ANSWER KEYSGonzalo CampNo ratings yet