Professional Documents
Culture Documents
Morphine Drug Study
Uploaded by
Xerxes Dejito100%(2)100% found this document useful (2 votes)
1K views3 pagesMorphine is a narcotic analgesic used to relieve severe pain. It works by binding to opioid receptors in the central nervous system and spinal cord. Common brand names include MS Contin and Avinza. It can be administered orally, intravenously, intramuscularly, subcutaneously, or epidurally. Side effects include respiratory depression, constipation, nausea, vomiting, and physical dependence with long-term use. Nurses must closely monitor patients for signs of respiratory depression and other adverse effects.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentMorphine is a narcotic analgesic used to relieve severe pain. It works by binding to opioid receptors in the central nervous system and spinal cord. Common brand names include MS Contin and Avinza. It can be administered orally, intravenously, intramuscularly, subcutaneously, or epidurally. Side effects include respiratory depression, constipation, nausea, vomiting, and physical dependence with long-term use. Nurses must closely monitor patients for signs of respiratory depression and other adverse effects.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
100%(2)100% found this document useful (2 votes)
1K views3 pagesMorphine Drug Study
Uploaded by
Xerxes DejitoMorphine is a narcotic analgesic used to relieve severe pain. It works by binding to opioid receptors in the central nervous system and spinal cord. Common brand names include MS Contin and Avinza. It can be administered orally, intravenously, intramuscularly, subcutaneously, or epidurally. Side effects include respiratory depression, constipation, nausea, vomiting, and physical dependence with long-term use. Nurses must closely monitor patients for signs of respiratory depression and other adverse effects.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 3
MORPHINE
Generic Name Morphine
Brand Name Astramorph PF,
Avinza, DepoDur,
Duramorph,
Epimorph , Kadian,
MSIR, MS Contin,
Oramorph SR,
Roxanol, RMS,
Statex
Classification CENTRAL NERVOUS SYSTEM (CNS) AGENT; ANALGESIC;
NARCOTIC (OPIATE) AGONIST
General Action Natural opium alkaloid with agonist activity by binding with the same
receptors as endogenous opioid peptides. Narcotic agonist effects are
identified with 3 types of receptors: Analgesia at supraspinal level,
euphoria, respiratory depression and physical dependence; analgesia at
spinal level, sedation and miosis; and dysphoric, hallucinogenic and
cardiac stimulant effects.
Dose and Route Pain Relief
Adult: PO 10–30 mg q4h prn or 15–30 mg sustained release q8–12h;
(Kadian) dose q12–24h, increase dose prn for pain relief; (Avinza) dose
q24h IV 2.5–15 mg q4h or 0.8–10 mg/h by continuous infusion, may
increase prn to control pain or 5–10 mg given epidurally q24h Epidural
(DepoDur only) 10–15 mg as single dose 30 min before surgery (max: 20
mg) IM/SC 5–20 mg q4h PR 10–20 mg q4h prn
Child: IV 0.05–0.1 mg/kg q4h or 0.025–2.6 mg/kg/h by continuous
infusion IM/SC 0.1–0.2 mg/kg q4h (max: 15 mg/dose) PO 0.2–0.5 mg/kg
q4–6h; 0.3–0.6 mg/kg sustained release q12h
Neonate: IV/IM/SC 0.05 mg/kg q4–8h (max: 0.1 mg/kg) or 0.01–0.02
mg/kg/h
Indications or Symptomatic relief of severe acute and chronic pain after nonnarcotic
Purposes analgesics have failed and as preanesthetic medication; also used to relieve
dyspnea of acute left ventricular failure and pulmonary edema and pain of
MI.
Side effects Body as a Whole: Hypersensitivity [Pruritus, rash, urticaria, edema,
hemorrhagic urticaria (rare), anaphylactoid reaction (rare)], sweating,
skeletal muscle flaccidity; cold, clammy skin, hypothermia.
CNS: Euphoria, insomnia, disorientation, visual disturbances, dysphoria,
paradoxic CNS stimulation (restlessness, tremor, delirium, insomnia),
convulsions (infants and children); decreased cough reflex, drowsiness,
dizziness, deep sleep, coma, continuous intrathecal infusion may cause
granulomas leading to paralysis.
Special Senses: Miosis.
CV: Bradycardia, palpitations, syncope; flushing of face, neck, and upper
thorax; orthostatic hypotension, cardiac arrest.
GI: Constipation, anorexia, dry mouth, biliary colic, nausea, vomiting,
elevated transaminase levels.
Urogenital: Urinary retention or urgency, dysuria, oliguria, reduced libido
or potency (prolonged use).
Other: Prolonged labor and respiratory depression of newborn.
Hematologic: Precipitation of porphyria.
Respiratory: Severe respiratory depression (as low as 2–4/min) or arrest;
pulmonary edema.
Contraindications Hypersensitivity to opiates; increased intracranial pressure; convulsive
disorders; acute alcoholism; acute bronchial asthma, chronic pulmonary
diseases, severe respiratory depression; chemical-irritant induced
pulmonary edema; prostatic hypertrophy; diarrhea caused by poisoning
until the toxic material has been eliminated; undiagnosed acute abdominal
conditions; following biliary tract surgery and surgical anastomosis;
pancreatitis; acute ulcerative colitis; severe liver or renal insufficiency;
Addison's disease; hypothyroidism; during labor for delivery of a
premature infant, in premature infants; pregnancy (category B; D in long-
term use or when high dose is used); lactation.
Nursing Assessment & Drug Effects
Responsibilities
Obtain baseline respiratory rate, depth, and rhythm and size of
pupils before administering the drug. Respirations of 12/min or
below and miosis are signs of toxicity. Withhold drug and report to
physician.
Observe patient closely to be certain pain relief is achieved. Record
relief of pain and duration of analgesia.
Be alert to elevated pulse or respiratory rate, restlessness, anorexia,
or drawn facial expression that may indicate need for analgesia.
Differentiate among restlessness as a sign of pain and the need for
medication, restlessness associated with hypoxia, and restlessness
caused by morphine-induced CNS stimulation (a paradoxic reaction
that is particularly common in women and older adult patients).
Monitor for respiratory depression; it can be severe for as long as
24 h after epidural or intrathecal administration.
Monitor carefully those at risk for severe respiratory depression
after epidural or intrathecal injection: Older adult or debilitated
patients or those with decreased respiratory reserve (e.g.,
emphysema, severe obesity, kyphoscoliosis).
Continue monitoring for respiratory depression for at least 24 h
after each epidural or intrathecal dose.
Assess vital signs at regular intervals. Morphine-induced
respiratory depression may occur even with small doses, and it
increases progressively with higher doses (generally max: 90 min
after SC, 30 min after IM, and 7 min after IV).
Encourage changes in position, deep breathing, and coughing
(unless contraindicated) at regularly scheduled intervals. Narcotic
analgesics also depress cough and sigh reflexes and thus may
induce atelectasis, especially in postoperative patients.
Be alert for nausea and orthostatic hypotension (with light-
headedness and dizziness) in ambulatory patients or when a supine
patient assumes the head-up position or in patients not experiencing
severe pain.
Monitor I&O ratio and pattern. Report oliguria or urinary retention.
Morphine may dull perception of bladder stimuli; therefore,
encourage the patient to void at least q4h. Palpate lower abdomen
to detect bladder distention.
Patient & Family Education
Avoid alcohol and other CNS depressants while receiving
morphine.
Do not use of any OTC drug unless approved by physician.
Do not smoke or ambulate without assistance after receiving drug.
Bedside rails are advised.
Use caution or avoid tasks requiring alertness (e.g., driving a car)
until response to drug is known since morphine may cause
drowsiness, dizziness, or blurred vision.
Do not breast feed while taking this drug.
You might also like
- Name of Drugs Indications Contraindications ADVERSE Reactions To Watch Out For Drug Interactions Nursing ConsiderationsDocument2 pagesName of Drugs Indications Contraindications ADVERSE Reactions To Watch Out For Drug Interactions Nursing ConsiderationsAlexis SilvestreNo ratings yet
- InsulinDocument2 pagesInsulinKristine YoungNo ratings yet
- Drug StudyDocument3 pagesDrug Studysnowyfingers100% (2)
- Difflam Drug StudyDocument1 pageDifflam Drug StudyDanlee EstandaNo ratings yet
- SenokotDocument1 pageSenokotKatie McPeek100% (1)
- Doctor's Order SampleDocument3 pagesDoctor's Order SampleXerxes DejitoNo ratings yet
- Morphine SulfateDocument5 pagesMorphine Sulfateapi-3797941100% (4)
- Losartan Drug StudyDocument2 pagesLosartan Drug StudyXerxes DejitoNo ratings yet
- Fentanyl Citrate Drug StudyDocument1 pageFentanyl Citrate Drug StudyArthur Christopher CorpuzNo ratings yet
- Drug Study - DigoxinDocument1 pageDrug Study - DigoxinSiergs Smith Gervacio100% (2)
- Nalbuphine (Nubain)Document2 pagesNalbuphine (Nubain)Adrianne Bazo100% (1)
- Pathophysiology of Varicella Zoster Virus-ChickenpoxDocument8 pagesPathophysiology of Varicella Zoster Virus-ChickenpoxXerxes DejitoNo ratings yet
- GabapentinDocument2 pagesGabapentinSar Patts100% (1)
- Drug Study Title Less Than 40 CharactersDocument2 pagesDrug Study Title Less Than 40 CharactersDan Mandig100% (1)
- Drug StudyDocument5 pagesDrug StudyLizeth Querubin93% (15)
- Drug StudyDocument9 pagesDrug StudyVicenia BalloganNo ratings yet
- Allopurinol Drug Study for Gout TreatmentDocument1 pageAllopurinol Drug Study for Gout TreatmentAbigail CastroNo ratings yet
- Nicardipine Drug StudyDocument3 pagesNicardipine Drug StudyXerxes Dejito50% (2)
- DRUG STUDY: MORPHINE SULFATE AND ITS USES, MECHANISM OF ACTION, EFFECTS, AND INTERACTIONSDocument8 pagesDRUG STUDY: MORPHINE SULFATE AND ITS USES, MECHANISM OF ACTION, EFFECTS, AND INTERACTIONSShara Lailanie A. Azis100% (1)
- NCP Risk For InfectionDocument3 pagesNCP Risk For InfectionXerxes Dejito0% (1)
- NCP Risk For Infection Secondary To Vehicular AccidentDocument2 pagesNCP Risk For Infection Secondary To Vehicular AccidentXerxes DejitoNo ratings yet
- Etiology and biological theories of schizophreniaDocument6 pagesEtiology and biological theories of schizophreniaXerxes DejitoNo ratings yet
- Drug Study ShenDocument12 pagesDrug Study ShenLass KazeNo ratings yet
- Loop Diuretic Furosemide Guide: Uses, Dosage, Side EffectsDocument2 pagesLoop Diuretic Furosemide Guide: Uses, Dosage, Side EffectsYou know whoNo ratings yet
- Dopamine Drug StudyDocument6 pagesDopamine Drug StudyGeorge RussellNo ratings yet
- Codeine Phosphate (Drug Study)Document2 pagesCodeine Phosphate (Drug Study)Franz.thenurse6888100% (2)
- AnalgesicDocument3 pagesAnalgesicAnnaMaeVelosoNo ratings yet
- MorphineDocument2 pagesMorphineNinoska Garcia-Ortiz80% (5)
- Aspirin: Generic NameDocument4 pagesAspirin: Generic NameGwww BabababaNo ratings yet
- DexmedetomidineDocument2 pagesDexmedetomidineapt48 ukwmsNo ratings yet
- Effects of atracurium besylateDocument3 pagesEffects of atracurium besylateWidya WidyariniNo ratings yet
- Indications and Usage: M M M M M M M M M M MDocument4 pagesIndications and Usage: M M M M M M M M M M MJesthony Lee CorderoNo ratings yet
- Drug - Htm#description.: Reference: Submitted By: Date Submitted: Submitted ToDocument2 pagesDrug - Htm#description.: Reference: Submitted By: Date Submitted: Submitted ToSHEILA MAE SACLOTNo ratings yet
- Noradrenaline (Norepinephrine) : 1mg/mLDocument5 pagesNoradrenaline (Norepinephrine) : 1mg/mLBrian RelsonNo ratings yet
- AtroventDocument2 pagesAtroventKatie McPeekNo ratings yet
- Epirubicin 10Document1 pageEpirubicin 10PdianghunNo ratings yet
- MetamucilDocument1 pageMetamucilSheri490No ratings yet
- Dopamine HydrochlorideDocument1 pageDopamine HydrochlorideJoannes SanchezNo ratings yet
- EsmololDocument2 pagesEsmololtherock316_995149No ratings yet
- Drug Study - VancomycinDocument2 pagesDrug Study - VancomycinKhatlen BagaresNo ratings yet
- DRUG STUDY LevetiracetamDocument3 pagesDRUG STUDY LevetiracetamMaria Althea NajorraNo ratings yet
- ZonisamideDocument2 pagesZonisamideRo-anne AkuNo ratings yet
- Drug StudyDocument2 pagesDrug StudyemmanuelmyagokayeNo ratings yet
- HaemaccelinfDocument9 pagesHaemaccelinfSisca YulistianaNo ratings yet
- PropranololDocument6 pagesPropranololanon_678895677No ratings yet
- Drug Study FinalDocument5 pagesDrug Study FinalJackie Ann Marie DapatNo ratings yet
- Assessment Nursing Diagnosis Planning Intervention Rationale EvaluationDocument2 pagesAssessment Nursing Diagnosis Planning Intervention Rationale EvaluationAbigail BascoNo ratings yet
- Drug StudyDocument2 pagesDrug StudyLee JennyNo ratings yet
- Cholestyramine (Drug Study)Document2 pagesCholestyramine (Drug Study)Franz.thenurse6888No ratings yet
- Filgrastim Boosts Neutrophil Recovery After ChemotherapyDocument3 pagesFilgrastim Boosts Neutrophil Recovery After ChemotherapyKyla Barrera TabungarNo ratings yet
- THEOPHYLLINE - Drug StudyDocument2 pagesTHEOPHYLLINE - Drug Studyeric macabiogNo ratings yet
- CefoperazoneDocument3 pagesCefoperazoneBaim FarmaNo ratings yet
- Metronidazole 500mg/tab 1 Tab 3xadayDocument4 pagesMetronidazole 500mg/tab 1 Tab 3xadayCrisyl LipawenNo ratings yet
- Atropine Drug ProfileDocument2 pagesAtropine Drug ProfileShahpmdNo ratings yet
- MIFEPREX™ (Mifepristone) Tablets, 200 MG For Oral Administration OnlyDocument16 pagesMIFEPREX™ (Mifepristone) Tablets, 200 MG For Oral Administration OnlyIniya RajendranNo ratings yet
- Drug Study: PART 1: To Be Completed Prior To Clinical ExperienceDocument5 pagesDrug Study: PART 1: To Be Completed Prior To Clinical ExperienceFrozanSNo ratings yet
- LacipilDocument2 pagesLacipilianecunarNo ratings yet
- AztreonamDocument2 pagesAztreonamHannahShaeHayesNo ratings yet
- Lowering Cholesterol with EzetimibeDocument2 pagesLowering Cholesterol with EzetimibeFranz Earl Niño AlbesaNo ratings yet
- CetirizineDocument1 pageCetirizineGabby Robles Paje100% (1)
- Drug Presentation On Morphine SulphateDocument38 pagesDrug Presentation On Morphine SulphateBHUVAN KUMAR. TNo ratings yet
- Name Classification Action Indication Adverse Effect Contraindication Nursing ConsiderationDocument2 pagesName Classification Action Indication Adverse Effect Contraindication Nursing ConsiderationSonny Dizon PareñasNo ratings yet
- Morphine SulfateDocument2 pagesMorphine SulfategreynabNo ratings yet
- Morphine Sulfate CNS AnalgesicDocument3 pagesMorphine Sulfate CNS AnalgesicSonny Dizon PareñasNo ratings yet
- Central Nervous System Drug StudyDocument11 pagesCentral Nervous System Drug StudySanny L Asim Jr.No ratings yet
- 11 15Document8 pages11 15Dinarkram Rabreca EculNo ratings yet
- PhenobarbitalDocument5 pagesPhenobarbitalapi-3797941100% (1)
- Ateneo de Davao University School of NursingDocument2 pagesAteneo de Davao University School of NursingXerxes DejitoNo ratings yet
- Weekly Requirement OB WardDocument12 pagesWeekly Requirement OB WardXerxes DejitoNo ratings yet
- Last Requirement OB WardDocument5 pagesLast Requirement OB WardXerxes DejitoNo ratings yet
- Readings in Philippine HistoryDocument1 pageReadings in Philippine HistoryXerxes DejitoNo ratings yet
- Aids PathoDocument1 pageAids PathoXerxes DejitoNo ratings yet
- Reading Re: Ortho CaseDocument1 pageReading Re: Ortho CaseXerxes DejitoNo ratings yet
- Psychopathophysiology of SchizophreniaDocument25 pagesPsychopathophysiology of SchizophreniaXerxes DejitoNo ratings yet
- Family Nursing Care PlanDocument2 pagesFamily Nursing Care PlanXerxes DejitoNo ratings yet
- SYMPTOMATOLOGY For CancerDocument12 pagesSYMPTOMATOLOGY For CancerXerxes DejitoNo ratings yet
- Reading About ORIF ProcedureDocument5 pagesReading About ORIF ProcedureXerxes DejitoNo ratings yet
- Procedural Report ORIFDocument4 pagesProcedural Report ORIFXerxes DejitoNo ratings yet
- Symptomatology SchizophreniaDocument8 pagesSymptomatology SchizophreniaXerxes DejitoNo ratings yet
- NCP Acute PainDocument2 pagesNCP Acute PainXerxes DejitoNo ratings yet
- Teaching Care PlanDocument3 pagesTeaching Care PlanXerxes DejitoNo ratings yet
- Drug StudyDocument6 pagesDrug StudyXerxes DejitoNo ratings yet
- Physical AssessmentDocument7 pagesPhysical AssessmentXerxes DejitoNo ratings yet
- Drug BankDocument6 pagesDrug BankXerxes DejitoNo ratings yet
- Reading 1-8Document4 pagesReading 1-8Xerxes DejitoNo ratings yet
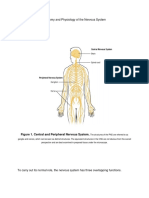
- Anatomy and Physiology of The Nervous SystemDocument34 pagesAnatomy and Physiology of The Nervous SystemXerxes DejitoNo ratings yet
- NCP Infection NewDocument3 pagesNCP Infection NewXerxes DejitoNo ratings yet
- NCP Infection NewDocument3 pagesNCP Infection NewXerxes DejitoNo ratings yet
- NCP Infection NewDocument3 pagesNCP Infection NewXerxes DejitoNo ratings yet