Professional Documents
Culture Documents
Valencesheet PDF
Valencesheet PDF
Uploaded by
Zia Muhammad HaiderOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Valencesheet PDF
Valencesheet PDF
Uploaded by
Zia Muhammad HaiderCopyright:
Available Formats
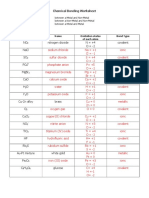
VALENCES OF SOME COMMON MAIN GROUP IONS
Cations Anions
+1 valence 1 valence
Li+1 = lithium F-1 = fluoride
Na+1 = sodium Cl-1 = chloride
K+1 = potassium Br-1 = bromide
Rb+1 = rubidium I-1 = iodide
Cs+1 = cesium
+2 valence 2 valence
Be+2 = beryllium O-2 = oxide
Mg+2 = magnesium S-2 = sulfide
Ca+2 = calcium
Sr+2 = strontium
Ba+2 = barium 3 valence
Sn+2 = tin(II) N-3 = nitride
Pb+2 = lead(II) P-3 = phosphide
+3 valence
Al+3 = aluminum
Ga+3 = gallium
+4 valence
Sn+4 = tin(IV)
Pb+4 = lead (IV)
VALENCES OF SOME COMMON TRANSITION GROUP IONS
Sc = +3
Ti = +2, +3, +4
V = +2, +3, +4, +5
Cr = +2, +3. +4, +5, +6
Mn = +2, +3, +4, +5, +6, +7
Fe = +2, +3, +4, +5, +6
Co = +2, +3
Ni = +2, +3, +4
Cu = +1, +2 Ag = +1
Zn = +2 Cd = +2 Hg = +1*, +2
*Mercury(I) is diatomic = Hg2+2 (each mercury ion has a +1 charge)
VALENCES OF SOME COMMON POLYATOMIC IONS
Cation
+1 valence
NH4+1 = ammonium
Anions
1 valence
C2H3O2-1 = acetate OH-1 = hydroxide
HCO3-1 = bicarbonate (or hydrogen carbonate) ClO-1 = hypochlorite
HSO4-1 = bisulfate (or hydrogen sulfate) NO3-1 = nitrate
HSO3-1 = bisulfite (or hydrogen sulfite) NO2-1 = nitrite
ClO3-1 = chlorate ClO4-1 = perchlorate
CN-1 = cyanide MnO4-1 = permanganate
2 valence
CO3-2 = carbonate SO4-2 = sulfate
CrO4-2 = chromate SO3-2 = sulfite
Cr2O7-2 = dichromate S2O3-2 = thiosulfate
3 valence
PO4-3 = phosphate
Prefixes for Binary Molecular Compounds
1 = mono 4 = tetra
2 = di 5 = penta
3 = tri 6 = hexa
You might also like
- Chemical Bonding-AnswersDocument2 pagesChemical Bonding-AnswersJaznMon71% (7)
- Ionic Puzzle ActivityDocument4 pagesIonic Puzzle ActivityEngr Mumtaz0% (1)
- 10 - Ionic Bonding ActivityDocument4 pages10 - Ionic Bonding Activityapi-292000448No ratings yet
- Representation of Some Positive Radicals and Their ValencyDocument2 pagesRepresentation of Some Positive Radicals and Their ValencyThePsychoNinjaNo ratings yet
- Information - Sheet - Merge 2Document3 pagesInformation - Sheet - Merge 2Frederick NakosNo ratings yet
- Valency and Formulae-HandoutDocument3 pagesValency and Formulae-HandoutABHAVYA RAJNo ratings yet
- Write The Formulas For The Following Ionic Compounds:: Bonding and Naming WS 4Document2 pagesWrite The Formulas For The Following Ionic Compounds:: Bonding and Naming WS 4Bea Lha Zandra BesingaNo ratings yet
- Common Ions and Their ChargesDocument1 pageCommon Ions and Their ChargesNonbinaryBroadwayNo ratings yet
- W3 02 Naming Chemical Formulas of CompoundsDocument20 pagesW3 02 Naming Chemical Formulas of CompoundsResmiel IrishNo ratings yet
- AP Chemistry Summer Assignment 2017Document44 pagesAP Chemistry Summer Assignment 2017John SmithNo ratings yet
- Element Group Cation Element Group AnionsDocument3 pagesElement Group Cation Element Group AnionsCharlotte TanNo ratings yet
- 872939cf-8b8a-4c72-902a-f9d3f2cfaf34Document9 pages872939cf-8b8a-4c72-902a-f9d3f2cfaf34Zynx DixonNo ratings yet
- NomenclatureDocument68 pagesNomenclatureel tetraNo ratings yet
- Ivan Zaman: Element (Metal) Symbol Valency Atomic Element Symbol Valency AtomicDocument2 pagesIvan Zaman: Element (Metal) Symbol Valency Atomic Element Symbol Valency AtomicJobayer Mahin100% (1)
- Symbols and Names For Common Polyatomic IonsDocument1 pageSymbols and Names For Common Polyatomic IonsElixirNo ratings yet
- List of Cations and AnionsDocument2 pagesList of Cations and AnionsArvin MagtotoNo ratings yet
- Table 1: Usual Oxidation Number of The Ions of Some Common ElementsDocument1 pageTable 1: Usual Oxidation Number of The Ions of Some Common Elementsliam leeNo ratings yet
- Naming Ionic CompoundsDocument17 pagesNaming Ionic CompoundsReena NasriNo ratings yet
- Chemical Nomenclature: (Naming Compounds)Document38 pagesChemical Nomenclature: (Naming Compounds)AhadSamiNo ratings yet
- Valence of Common Ions and RadicalsDocument3 pagesValence of Common Ions and RadicalsFrederick FranciscoNo ratings yet
- Chemical NomenclatureDocument7 pagesChemical NomenclatureKeith Lavin100% (1)
- Common Ion ChargesDocument1 pageCommon Ion ChargesMoganan SubramaniamNo ratings yet
- Polyatomic Ions: +1 Cations - 2 AnionsDocument2 pagesPolyatomic Ions: +1 Cations - 2 AnionsAngelica Nehmyah CuevasNo ratings yet
- Polyatomic Ion Master ListDocument1 pagePolyatomic Ion Master ListAldrin Morallos MaglahusNo ratings yet
- Ch12 Redox Ws Keys 1 13Document28 pagesCh12 Redox Ws Keys 1 13Allen IBARRA VILLAMINNo ratings yet
- Names of RadicalsDocument4 pagesNames of RadicalsSnehin PoddarNo ratings yet
- Cations - Anions AlphabeticalDocument1 pageCations - Anions AlphabeticalNP100% (3)
- Module 3 - Atoms and MoleculesDocument13 pagesModule 3 - Atoms and Moleculesagrawalrashmi2177No ratings yet
- Write The Name of The Derived FormulaDocument1 pageWrite The Name of The Derived Formulaמתבונן בך אלוהיםNo ratings yet
- Chemistry Class 10 Lecture 1 (3 April)Document5 pagesChemistry Class 10 Lecture 1 (3 April)allroundersaksham304No ratings yet
- Valencies of Ions (For 9th and 10th)Document6 pagesValencies of Ions (For 9th and 10th)Irene AbhilashNo ratings yet
- Lecture 1 EquationsDocument11 pagesLecture 1 Equationsmerabamoding11No ratings yet
- Naming Compounds Cheat SheetDocument2 pagesNaming Compounds Cheat SheetYSA BELLENo ratings yet
- Chapter 5 Coordination CompoundDocument36 pagesChapter 5 Coordination Compoundammar zakariaNo ratings yet
- Naming CompoundsDocument2 pagesNaming CompoundsTeresa Marie CorderoNo ratings yet
- Table of Polyatomic Ion1Document3 pagesTable of Polyatomic Ion1Borndis WayNo ratings yet
- Cations: Ions and Charges Cations With Multiple ChargesDocument1 pageCations: Ions and Charges Cations With Multiple ChargesJohn Rey BayoguingNo ratings yet
- Review Material For Exam IDocument5 pagesReview Material For Exam IquimicosorioNo ratings yet
- General Chemistry 2 Module 3Document6 pagesGeneral Chemistry 2 Module 3Jason Vinluan CarinanNo ratings yet
- Oxidation NumberDocument14 pagesOxidation Numbermysha moontahaNo ratings yet
- Chemical Formula Writing Worksheet PDFDocument4 pagesChemical Formula Writing Worksheet PDFkezia0% (1)
- Ions and Their Charges: Metals With Variable Oxidation NumbersDocument1 pageIons and Their Charges: Metals With Variable Oxidation Numbers123 123No ratings yet
- Ion Sheet With Solubility Rules-2Document1 pageIon Sheet With Solubility Rules-2kwilsonNo ratings yet
- Formulae of Common IonsDocument1 pageFormulae of Common IonsJoel OkohNo ratings yet
- Advanced-Chem Q1 LP7Document6 pagesAdvanced-Chem Q1 LP7Francesca BuenoNo ratings yet
- Brand Pitch Presentation in Magenta Orange Cream Bold Modern StyleDocument3 pagesBrand Pitch Presentation in Magenta Orange Cream Bold Modern StyleDanishNo ratings yet
- Most Elements' Valency NESDocument2 pagesMost Elements' Valency NESHakim AbbasNo ratings yet
- Naming and Formula WritingDocument15 pagesNaming and Formula WritingMeggy CaparasNo ratings yet
- Week 7 Redox Reactions Primitive DefinitionsDocument5 pagesWeek 7 Redox Reactions Primitive DefinitionsTunde DabiriNo ratings yet
- Abbigale Leckington - Ionic - Compound - PuzzleDocument5 pagesAbbigale Leckington - Ionic - Compound - Puzzle992499021No ratings yet
- Sarah Compound Dice ActivityDocument3 pagesSarah Compound Dice ActivityAnaria ManojNo ratings yet
- ionicChargesChart PDFDocument1 pageionicChargesChart PDFronit675No ratings yet
- Ion Memorization ListDocument2 pagesIon Memorization Listdchao94No ratings yet
- Compound FormationDocument7 pagesCompound Formationniteshhacker3372No ratings yet
- Chemical Elements Pocket Guide: Detailed Summary of the Periodic TableFrom EverandChemical Elements Pocket Guide: Detailed Summary of the Periodic TableNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
- The Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyFrom EverandThe Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyNo ratings yet