Professional Documents
Culture Documents
Chapter-5 Results and Discussion PDF
Uploaded by
Sunil ChaudharyOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter-5 Results and Discussion PDF
Uploaded by
Sunil ChaudharyCopyright:
Available Formats
CHAPTER-5 RESULTS AND DISCUSSION
RESULTS
Melting point:
Melting point of Montelukast Sodium was determined by capillary method and results fount
to be 108-110˚ C, which complied with the standard monograph , indicating the purity of the
drug.
Solubility analysis:
Montelukast Sodium found to be soluble in water, ethanol and dimethyl formamide
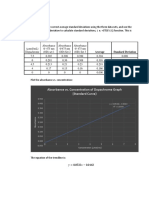
Calibration curve of Montelukast Sodium:
Montelukast Sodium obey the Beer’s law in concentration range of 5-30 µg/ml in 0.5%
SLS with regression coefficients (R2) of 0.9987. The calibration data is given table and
calibration curve was constructed in graph no.1
Table No. 10: Calibration Curve of Montelukast Sodium in 240 nm.
S.N. Concentration (µg/ml) Absorbance (240nm)
1 0 0±0.00
2 5 0.156±0.015
3 10 0.293±0.001
4 15 0.460±0.012
5 20 0.583±0.016
6 25 0.769±0.062
7 30 0.898±0.014
Note: All values are expressed as mean ± SD. n=3.
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 47
CHAPTER-5 RESULTS AND DISCUSSION
1
0.9
0.8
0.7
y = 0.0301x
0.6 R² = 0.9987
Absorbance
0.5
0.4
0.3
0.2
0.1
0
0 5 10 15 20 25 30 35
Concentration(µg/ml)
Graph No.1: Calibration curve of Montelukast Sodium at 240 nm
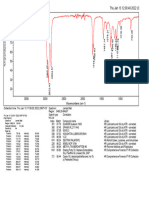
Drug and polymer compatibility studies
Drug and polymers are used to prepare oral dispersible tablet were checked for
compatibility study by carrying out FTIR spectroscopy. The FTIR spectra obtained for pure
drug and drug-polymers mixture from 4000 to 400 cm-1 are given as follows.
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 48
CHAPTER-5 RESULTS AND DISCUSSION
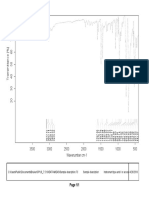
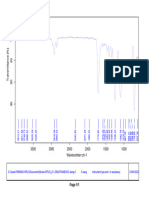
Fig. No.9: FTIR spectra of Montelukast Sodium
100 95
Transmittance [%]
90 85
80
3850.41
2910.50
1633.79
1556.16
1495.03
1393.09
1219.80
1130.08
1067.11
1016.67
960.62
928.80
861.82
834.70
758.62
695.18
593.82
561.11
526.81
514.47
3500 3000 2500 2000 1500 1000 500
Wavenumber cm-1
C:\Program Files\OPUS_65\MEAS\Montelukast Sodium.0 Montelukast Sodium ATR eco ZnSe 24/01/2019
Page 1/1
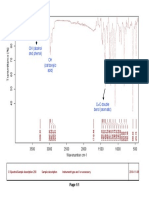
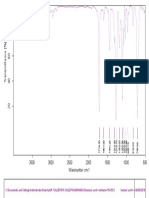
Fig. No. 10: FTIR spectra of Montelukast Sodium+ Crospovidone
100 95
Transmittance [%]
90 85
80
3268.37
1650.96
1556.14
1494.83
1417.49
1287.57
1129.62
1066.87
960.75
835.00
749.50
695.23
566.66
530.83
513.97
3500 3000 2500 2000 1500 1000 500
Wavenumber cm-1
C:\Program Files\OPUS_65\MEAS\Montelukast Sodium + Crosspovidone.0 Montelukast Sodium + Crosspovidone ATR eco ZnSe 24/01/2019
Page 1/1
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 49
CHAPTER-5 RESULTS AND DISCUSSION
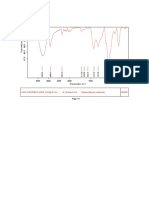
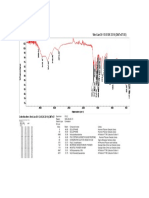
Fig. No. 11: FTIR spectra of Montelukast Sodium + Sodium Starch Glycolate
100 95
Transmittance [%]
90 85
80
3850.24
3732.37
3646.10
3248.68
1591.99
1556.42
1495.10
1404.46
1144.79
997.35
927.44
861.39
834.86
759.35
694.49
562.02
526.85
518.93
510.08
3500 3000 2500 2000 1500 1000 500
Wavenumber cm-1
C:\Program Files\OPUS_65\MEAS\Montelukast Sodium + Sodium starch glycolate.0 Montelukast Sodium + Sodium starch glycolate ATR
24/01/2019
eco ZnSe
Page 1/1
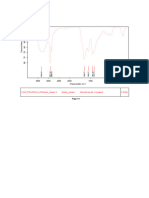
Fig. No.12: FTIR spectra of Montelukast Sodium +Guar Gum
100
99
Transmittance [%]
97 96
9598
3893.32
3844.28
3827.21
3742.56
3677.09
3641.58
3245.33
2918.57
2358.49
2311.58
2130.39
1740.55
1642.35
1547.92
1493.15
1393.62
1243.78
1135.79
1061.07
1013.40
961.06
865.06
829.72
753.48
688.62
606.93
554.02
522.35
3500 3000 2500 2000 1500 1000 500
Wavenumber cm-1
C:\Program Files\OPUS_65\MEAS\Montelukast + Guar gum.0 solid ATR eco ZnSe 20/12/2018
Page 1/1
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 50
CHAPTER-5 RESULTS AND DISCUSSION
Fig .No. 13: FTIR spectra of Montelukast Sodium + Gellan Gum
100
98 96
Transmittance [%]
90 92 94
88
86
3231.06
2923.32
1547.24
1491.64
1394.34
1013.00
961.36
866.19
832.00
751.26
570.54
3500 3000 2500 2000 1500 1000 500
Wavenumber cm-1
C:\Program Files\OPUS_65\MEAS\Montelukast sodium + Gallen gum.0 solid ATR eco ZnSe 20/12/2018
Page 1/1
Fig .No. 14: FTIR spectra of Montelukast Sodium + Sterculia Gum
100
98 96
Transmittance [%]
90 92 94
88
86
3749.17
3229.20
2924.08
1592.74
1556.58
1495.22
1393.65
1011.69
964.07
835.27
571.56
552.36
523.07
3500 3000 2500 2000 1500 1000 500
Wavenumber cm-1
C:\Program Files\OPUS_65\MEAS\Montelukast sodium + Sterculia gum.0 solid ATR eco ZnSe 20/12/2018
Page 1/1
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 51
CHAPTER-5 RESULTS AND DISCUSSION
Fig .No. 15: FTIR spectra of Montelukast Sodium + Cross povidone + Guar Gum
100
95
Transmittance [%]
90 85
80
3850.79
2894.33
1644.91
1556.71
1494.87
1462.37
1421.79
1288.01
1142.76
1014.11
864.22
835.46
577.52
566.07
540.78
531.46
516.44
504.11
3500 3000 2500 2000 1500 1000 500
Wavenumber cm-1
C:\Program Files\OPUS_65\MEAS\Montelukast sodium + Cross povidone + Guar gum.0 solid ATR eco ZnSe 20/12/2018
Page 1/1
Fig .No. 16: FTIR spectra of Montelukast Sodium + Cross povidone + Gellan Gum
100
98 96
Transmittance [%]
90 92 94
88
86
3851.20
3732.91
3686.98
3646.86
3242.08
2920.37
1644.82
1606.94
1556.88
1495.41
1417.38
1288.27
1018.61
963.78
834.26
612.74
548.33
533.14
519.02
509.58
3500 3000 2500 2000 1500 1000 500
Wavenumber cm-1
C:\Program Files\OPUS_65\MEAS\Montelukast sodium + Cross povidone + Gallen gum.0 solid ATR eco ZnSe 20/12/2018
Page 1/1
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 52
CHAPTER-5 RESULTS AND DISCUSSION
Fig .No. 17: FTIR spectra of Montelukast Sodium + Cross povidone + Sterculia Gum
100
98 96
Transmittance [%]
90 92 94
88
86
3850.56
3742.13
3281.51
2916.51
1644.68
1556.51
1495.14
1418.21
1288.01
1017.08
964.96
835.14
585.21
557.59
534.73
527.81
516.29
509.77
3500 3000 2500 2000 1500 1000 500
Wavenumber cm-1
C:\Program Files\OPUS_65\MEAS\Montelukast sodium + Cross povidone + Sterculia gum.0 solid ATR eco ZnSe 20/12/2018
Page 1/1
Fig .No. 18: FTIR spectra of Montelukast Sodium + Sodium Starch Glycolate
+ Guar Gum
100
98
96
Transmittance [%]
90 92 88
86
8494
3898.55
3882.46
3851.43
3835.92
3815.65
3800.06
3748.31
3709.81
3687.09
3646.62
3626.56
3275.66
2915.59
2351.62
2337.16
1731.62
1660.24
1644.54
1633.86
1592.53
1574.46
1556.82
1538.91
1495.51
1404.68
1337.10
1145.37
999.01
862.69
834.66
759.93
693.44
666.57
619.60
592.62
562.28
547.83
528.24
515.88
506.24
3500 3000 2500 2000 1500 1000 500
Wavenumber cm-1
C:\Program Files\OPUS_65\MEAS\Montelukast sodium + Sodium starch glycolate + Guar gum.0 solid ATR eco ZnSe 20/12/2018
Page 1/1
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 53
CHAPTER-5 RESULTS AND DISCUSSION
Fig.No. 19: FTIR spectra of Montelukast Sodium + Sodium Starch Glycolate
+ Gellan Gum
100
98 96
Transmittance [%]
90 92 94
88
86
3266.50
1593.11
1404.99
1001.71
516.24
3500 3000 2500 2000 1500 1000 500
Wavenumber cm-1
C:\Program Files\OPUS_65\MEAS\Montelukast sodium + Sodium starch glycolate + Gallen gum.0 solid ATR eco ZnSe 20/12/2018
Page 1/1
Fig.No. 20: FTIR spectra of Montelukast Sodium + Sodium Starch Glycolate
+ Sterculia Gum
100
98
Transmittance [%]
94 96
92
90
3229.09
1592.41
1495.52
1404.58
998.95
836.38
587.63
550.02
531.74
3500 3000 2500 2000 1500 1000 500
Wavenumber cm-1
C:\Program Files\OPUS_65\MEAS\Montelukast sodium + Sodium starch glycolate + Sterculia gum.0 solid ATR eco ZnSe 20/12/2018
Page 1/1
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 54
CHAPTER-5 RESULTS AND DISCUSSION
Table No. 11: FTIR position of characteristic bond vibrations of pure drug Montelukast
Sodium and the drug with excipients.
S. Various Mode Observed FTIR Positions of various Bond-vibrations in Wavenumber (cm-1)
N of Bond
Pure Drug+Cro Drug+ Drug+Guar Drug+Gella Drug+Ste
vibrations and
drug spovidone SSG .G n. G r. G
Wavenumber
Range (cm-1)
1. N-H Stretching 3245.33 3268.37 3248.68 3245.33 3231.06 3229.20
(3000-3700)
2. C-H Stretching 2910.50 2910.50 2915.25 2918.57 2923.32 2924.08
(2700-3300)
3. C=O 1556.16 1650.96 1591.99 1642.35 1547.24 1592.74
Stretching
(1600-1900)
4. C-N Stretching 1130.08 1287.57 1144.79 1135.79 1145.55 1235.17
(1080-1360)
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 55
CHAPTER-5 RESULTS AND DISCUSSION
Table No. 12: FTIR position of characteristic bond vibrations of pure drug Montelukast
Sodium and the drug with excipients.
S. Various Mode Observed FTIR Positions of various Bond-vibrations in Wavenumber (cm-1)
N of Bond
vibrations and Drug+Cr Drug+Cros. Drug+Cro Drug+SSG. Drug+SSG Drug+SSG
Wavenumber os+Guar +Gellan G s.+ Ster. G +Guar G +Gellan G +Ster. G
-1
Range (cm ) G
1. N-H Stretching 3189.56 3242.08 3281.51 3275.66 3266.50 3229.09
(3000-3700)
2. C-H Stretching 2894.33 2920.37 2916.51 2915.59 2915.59 2925.50
(2700-3300)
3. C=O 1644.911 1644.82 1644.68 1644.54 1593.11 1592.42
Stretching
(1600-1900)
4. C-N Stretching 1142.76 1288.27 1288.01 1145.37 1142.76 1142.76
(1080-1360)
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 56
CHAPTER-5 RESULTS AND DISCUSSION
EVALUATION OF TABLETS OF ALL FORMULATIONS
Table no 13: Pre-compression parameters of Montelukast Sodium powder mixture
Formulation Bulk density Tapped density Carr’s Index Hausner’s Angle of
Batch (gm/cm3) (gm/cm3) (%) ratio repose (°)
F1 0.315±0.003 0.358±0.004 12.01±0.76 1.13±0.008 27.30±1.13
F2 0.303±0.001 0.342±0.006 11.40±0.14 1.12±0.003 28.20±1.08
F3 0.312±0.006 0.363±0.002 14.04±0.04 1.16±0.012 29.80±1.19
F4 0.310±0.003 0.359±0.003 13.64±0.06 1.15±0.007 27.90±1.08
F5 0.319±0.012 0.367±0.004 13.07±0.15 1.15±0.004 28.50±0.98
F6 0.315±0.014 0.361±0.003 12.74±0.21 1.14±0.016 28.90±1.16
F7 0.302±0.021 0.355±0.002 14.92±0.09 1.17±0.014 28.10±0.05
F8 0.304±0.006 0.354±0.005 14.12±0.03 1.16±0.006 27.70±0.47
F9 0.318±0.014 0.373±0.002 14.74±0.02 1.17±0.006 29.20±1.39
F10 0.306±0.012 0.357±0.006 14.28±0.06 1.16±0.005 29.70±1.56
F11 0.315±0.006 0.358±0.007 12.01±0.07 1.13±0.012 29.90±1.23
F12 0.309±0.004 0.356±0.002 13.20±0.14 1.15±0.009 28.10±1.12
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 57
CHAPTER-5 RESULTS AND DISCUSSION
F13 0.317±0.014 0.371±0.005 14.55±0.09 1.17±0.007 27.80±1.17
F14 0.313±0.015 0.365±0.006 14.24±0.16 1.16±0.005 28.40±1.08
F15 0.308±0.007 0.362±0.005 14.91±0.08 1.17±0.008 29.10±1.13
F16 0.303±0.012 0.344±0.002 11.91±0.04 1.13±0.012 28.50±0.08
F17 0.318±0.003 0.374±0.003 14.97±0.18 1.17±0.006 27.70±1.31
F18 0.315±0.008 0.363±0.004 13.33±0.11 1.15±0.003 29.40±1.19
Note: All values are expressed as mean ± SD. n=3.
Table No.14: Post-compression evaluations of Montelukast Sodium tablet
Formulation Wt. Hardness Thickness Friability Drug Disintegration
Batch variation (kg/ cm2) (mm) (%) Content time
(mg) n=10 (%) (Seconds)
F1 179.4±0.07 3.0±0.20 3.0±0.03 0.33±0.02 98.0±1.02 21±0.04
F2 180.0±1.20 3.0±0.30 3.0±0.02 0.35±0.03 95.0±1.03 14±0.08
F3 179.6±0.30 3.5±0.20 3.4±0.11 0.31±0.02 97.0±1.05 09±0.20
F4 179.2±0.09 3.5±0.18 3.4±0.20 0.31±0.16 97.0±1.05 30±0.05
F5 180.5±0.06 3.5±0.12 3.4±0.08 0.32±0.12 98.0±1.10 36±0.05
F6 181.5±0.08 3.5±0.20 3.4±0.15 0.33±0.11 100±1.02 31±0.10
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 58
CHAPTER-5 RESULTS AND DISCUSSION
F7 179.3±2.00 3.6±0.17 3.2±0.30 0.34±0.17 96.0±1.04 18±1.02
F8 181.0±1.20 3.6±0.40 3.2±0.60 0.35±1.02 95.0±0.06 17±1.04
F9 179.8±1.00 3.6±0.13 3.2±1.08 0.35±0.09 91.0±0.08 22±0.20
F10 181.1±0.60 3.2±1.20 3.8±0.70 0.34±0.10 93.6±1.04 18±1.06
F11 180.7±1.04 3.0±1.04 3.5±0.09 0.32±0.16 92.0±0.05 13±1.02
F12 178.8±0.30 3.5±0.80 3.5±0.16 0.32±0.03 96.8.±0.10 16±0.06
F13 179.4±0.50 3.5±0.50 3.5±0.40 0.31±0.08 96.0±0.06 40±0.12
F14 180.4±1.02 3.5±0.18 3.6±0.40 0.34±0.11 96.2±1.06 25±1.05
F15 178.6±1.00 3.5±1.15 3.6±0.05 0.32±0.05 95.3±1.02 19±0.16
F16 180.0±0.06 3.5±0.12 3.6±0.15 0.31±0.14 92.2±0.08 21±0.09
F17 179.3±0.07 3.5±1.13 3.6±0.65 0.35±0.17 106±0.09 39±0.10
F18 179.8±0.30 3.5±0.50 3.6±0.15 0.33±0.07 102±1.02 32±1.02
Note: All values are expressed as mean ± SD. n=3
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 59
CHAPTER-5 RESULTS AND DISCUSSION
Pre-compressional evaluation parameters:
Results of the pre-compressional parameters performed on the powder blend for tablets
formulations F1 to F18 are reported in the table no.11. The angle of repose for all the
formulations (F1 – F18) was found to be within the range of 27.30 ± 1.13 to 29.90 ± 1.23,
showing good flow characteristics. Hausner’s ratio was found to be in the range of 1.12 ±
0.003 to 1.17 ± 0.006 and compressibility index was found to be in the range of 11.40 ±
0.14to 14.97 ± 0.18, indicating good flow ability of the tablet formulations.
Post-compression parameters
Shape and appearance:
White colour, round flat uncoated tablets having one side breakline.
Weight Variation:
Prepared tablets of all formulations were evaluated for weight variation and standard
deviations from the average weight are reported in table no.12. The average weights of all
the formulations (F1 to F18) were within the range of 179.2 + 0.09 to 181.5 + 0.08. All
the tablets passed the weight variation test, i.e., the average percentage weight variation
was found to be within the prescribed pharmacopoeia limits of ±7.5%.
Tablet Thickness and Hardness:
The thickness of the tablets from batch F1 to F18 was found to be between 3.0 ± 0.03 to
3.8 ± 0.70 mm and hardness was found to be within the range of 3.0 ± 0.20 to 3.6 ± 0.50
Kg/cm2 as reported in table no.12. The low standard deviation values indicate that the
thicknesses as well as hardness of all the formulations were almost uniform and also the
tablets possess good mechanical strength with sufficient hardness.
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 60
CHAPTER-5 RESULTS AND DISCUSSION
Friability:
The friability of the formulations (F1 to F18) was found to be between 0.31±0.02% to
0.35±1.02% as reported in table no.12. The obtained results were found to be within the
range (<1%) in all the formulations indicating tablets possess good mechanical strength.
Drug Content Uniformity:
The Percentage of drug content for F1 to F18 was found to be in the range of 91.0 ±
0.05% to 106.0 ± 0.09 % as shown in table no.12. The results were within the limit (not
< 90% and not > 110%) as specified in Indian pharmacopeia.
Disintegration Time:
The disintegration time for dispersible tablets F1 to F18 is shown in table no.12. The in-
vitro disintegration time was found to be very less for F3 formulation that is 09 + 0.20
seconds. As the concentration of super disintegrants increases, there is decrease in the
disintegration time.
In-Vitro Dissolution Studies:
The cumulative percentage drug released from each tablet formulation was studied at
different time intervals. The dissolution profile for formulation F1 to F18 is shown in
table no.21. and graph no.10. Formulation F9 shown better dissolution profile when
compared to remaining formulations.
Drug Release Kinetics:
The mechanism of drug release from formulation F9 were characterized by zero order
kinetics, first order kinetics, Higuchi’s kinetics, Korsmeyer-Peppas model plots as ore shown
graph 10. It was observed that the high correlation coefficient (R2) was found to be 1. first
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 61
CHAPTER-5 RESULTS AND DISCUSSION
order kinetics, which indicates the drug release rate of fast dissolving tablets would be
dependent of its concentration.
Stability Studies:
From the stability studies, it was clear that the formulation were physically and
chemically stable for 90 days. And there was no significant change in the physical
parameters, drug content and in-vito dissolution release profiles.
Department of Pharmaceutics, Mallige college of pharmacy, Bangalore Page 62
You might also like
- Chapter-5 Results and DiscussionDocument17 pagesChapter-5 Results and DiscussionSunil ChaudharyNo ratings yet
- Beth 2 PDFDocument1 pageBeth 2 PDFAustin/KenzieNo ratings yet
- IR Spectros PDFDocument1 pageIR Spectros PDFAylin MansimovaNo ratings yet
- G-D-K-A-K-V-C-A - AcetaminofenDocument1 pageG-D-K-A-K-V-C-A - AcetaminofenAndres AriasNo ratings yet
- EugenolDocument4 pagesEugenolRandeNo ratings yet
- 7001 - Nhua Chau AuDocument1 page7001 - Nhua Chau AuThanh Linh NguyenNo ratings yet
- F-chm4301 (DR Haslina)Document1 pageF-chm4301 (DR Haslina)MUNA AIREENI BINTI MUSA MUSLI / UPMNo ratings yet
- LE 40 Group A - Sample PVADocument1 pageLE 40 Group A - Sample PVAÑojib Ëasar ProttoyNo ratings yet
- Shahriar - Sample 1Document1 pageShahriar - Sample 1alshahriar1313No ratings yet
- Experiment 6 Data2Document4 pagesExperiment 6 Data2Emmanuel PlazaNo ratings yet
- Tridecanal 4graden 14112022Document1 pageTridecanal 4graden 14112022Abdelhamid TahriNo ratings yet
- 3 SiangDocument1 page3 SiangAidatul FitriyahNo ratings yet
- Date/Time 6/5/2022 3:49:25 PM No. of Scans Resolution Comment G-D-K-A-K-V-C-A-StandarDocument1 pageDate/Time 6/5/2022 3:49:25 PM No. of Scans Resolution Comment G-D-K-A-K-V-C-A-StandarAndres AriasNo ratings yet
- Problem 13.29.: Chap13 Supplement 2Document5 pagesProblem 13.29.: Chap13 Supplement 2Tung HarryNo ratings yet
- Abs Lemak BabiDocument1 pageAbs Lemak BabiSanty SetiaNingsihNo ratings yet
- Donny Ivananda - 1941420075 - 01 Data Distilasi-2Document7 pagesDonny Ivananda - 1941420075 - 01 Data Distilasi-2Lorenz simamoraNo ratings yet
- Benzoic AcidDocument2 pagesBenzoic AcidmmmNo ratings yet
- REP35MDocument1 pageREP35MMauricio SanchezNo ratings yet
- 3 PagiDocument1 page3 PagiAidatul FitriyahNo ratings yet
- Benzoic Acid+ Methanol-154-55Document1 pageBenzoic Acid+ Methanol-154-55Shreeyesh BiswalNo ratings yet
- Tabela 3 0.5 BarDocument1 pageTabela 3 0.5 BarAna BalbiNo ratings yet
- 1 s2.0 S022352341530180X mmc1Document80 pages1 s2.0 S022352341530180X mmc1tumtumNo ratings yet
- 02 K Charts - AE+SIDocument1 page02 K Charts - AE+SILalang Dwiyoga SaktiNo ratings yet
- 220213 Плочасти - детаљно само GDocument94 pages220213 Плочасти - детаљно само GTomoNo ratings yet
- Dispurger Motor Starting PlotsDocument8 pagesDispurger Motor Starting PlotsRitish AmalNo ratings yet
- PDF DetailDocument1 pagePDF Detailelsieazcuna024No ratings yet
- PDF DetailDocument1 pagePDF DetailKaiser CarloNo ratings yet
- Grafica 2Document2 pagesGrafica 2Elmer Santisteban SanchezNo ratings yet
- Seguimiento A La Fermentación Tiempo (Horas) Absorbancia Sólidos Solubles % PPM Lev Dilución G/L Levadura 0Document3 pagesSeguimiento A La Fermentación Tiempo (Horas) Absorbancia Sólidos Solubles % PPM Lev Dilución G/L Levadura 0Alejo MuñozNo ratings yet
- 1 123041Document1 page1 123041JG DessignNo ratings yet
- % Weight (Sample) Graph: Screen Size (MM)Document5 pages% Weight (Sample) Graph: Screen Size (MM)Avian Tri KuncoroNo ratings yet
- Che501 HWDocument2 pagesChe501 HWCamila Shaine BelmonteNo ratings yet
- 0506 (CCHICA) 33KV: 2Lprch 2LprchDocument1 page0506 (CCHICA) 33KV: 2Lprch 2LprchJIMENEZPSNo ratings yet
- 27-2-Q - Sieve TestDocument15 pages27-2-Q - Sieve TestWafik AlhajiNo ratings yet
- Goal Seek to find time for 95% jet speedDocument1 pageGoal Seek to find time for 95% jet speedRamón RamalhoNo ratings yet
- %T Lemak BabiDocument1 page%T Lemak BabiSanty SetiaNingsihNo ratings yet
- Indoor Luminaire Photometric Data: IES Indoor Report Photometric Filename:AR70 GU10-2.IESDocument13 pagesIndoor Luminaire Photometric Data: IES Indoor Report Photometric Filename:AR70 GU10-2.IESGonzalo LunaNo ratings yet
- SieveDocument3 pagesSieveWafik AlhajiNo ratings yet
- AGGREGATE TESTINGDocument15 pagesAGGREGATE TESTINGSubodh Kumar KamalNo ratings yet
- Additive AravinthDocument15 pagesAdditive AravinthAravinth subramanianNo ratings yet
- پارتیکل سایزDocument1 pageپارتیکل سایزsaeid.mehregan.shNo ratings yet
- Flood Luminaire Photometric Data: IES Flood Report Photometric Filename:724574 LG179 10W.IESDocument16 pagesFlood Luminaire Photometric Data: IES Flood Report Photometric Filename:724574 LG179 10W.IESGonzalo LunaNo ratings yet
- Lab AnalysisDocument4 pagesLab AnalysisErnestasBlaževičNo ratings yet
- ASCORBIC ACID Abs (1) TRDocument1 pageASCORBIC ACID Abs (1) TRSuleiman MohammedNo ratings yet
- Monitoring Acid-Catalyzed Hydrolysis of Sucrose Through Low Resolution NMR RelaxometryDocument25 pagesMonitoring Acid-Catalyzed Hydrolysis of Sucrose Through Low Resolution NMR Relaxometryfaizfrasat123No ratings yet
- Dynamic Head: Gr.A L-210 (4.9 MM) Gr.A L-210 (4.9 MM) Gr.A L-210 (4.9 MM) IS:1239 GI-H IS:1239 GI-M IS:1239 GI-MDocument3 pagesDynamic Head: Gr.A L-210 (4.9 MM) Gr.A L-210 (4.9 MM) Gr.A L-210 (4.9 MM) IS:1239 GI-H IS:1239 GI-M IS:1239 GI-MChauhan UjjvalNo ratings yet
- 2016 EXAM SPM Analisis Post MortermDocument1 page2016 EXAM SPM Analisis Post MortermyusmahairiNo ratings yet
- Concentration Vs Conductivity: 1.0 Results, Discussion and Analysis 1.1 Calibration CurveDocument9 pagesConcentration Vs Conductivity: 1.0 Results, Discussion and Analysis 1.1 Calibration CurveAhZaiSkyNo ratings yet
- Angular Gripper CT-40M-RE 180 Degree SeriesDocument1 pageAngular Gripper CT-40M-RE 180 Degree SeriesBe HappyNo ratings yet
- ThicknessDocument1 pageThicknessMaged Lotfy Abdel-aalNo ratings yet
- SEM Images and FTIR Analysis of SS316LDocument13 pagesSEM Images and FTIR Analysis of SS316Lkishor reddyNo ratings yet
- Libro 1Document3 pagesLibro 1Kmby GabrielaNo ratings yet
- Density (G/CM ) Against Oil Composition (WT%) : 0.99 1 F (X) - 0.0008654545x + 0.9897272727 R 0.9595189193Document5 pagesDensity (G/CM ) Against Oil Composition (WT%) : 0.99 1 F (X) - 0.0008654545x + 0.9897272727 R 0.9595189193Fhaliesha ZakurNo ratings yet
- Figure M 39 Ethanol Water MixtureDocument6 pagesFigure M 39 Ethanol Water MixtureHamza ShafiqNo ratings yet
- Axial FanDocument1 pageAxial FanKarimAboelmkaremNo ratings yet
- Fisa RoomTempSensorDocument2 pagesFisa RoomTempSensorSTEFANALINLUCIANNo ratings yet
- FTIR Spectrum of Sample-VS CuDocument1 pageFTIR Spectrum of Sample-VS CuRevathiNo ratings yet
- Agilent Resolutions Pro: Ethyl OleateDocument1 pageAgilent Resolutions Pro: Ethyl OleateanilNo ratings yet
- CEE/CNE 353: Civil Engineering Materials Solutions To Selected Problems in Homework - 3Document3 pagesCEE/CNE 353: Civil Engineering Materials Solutions To Selected Problems in Homework - 3Asad YousafNo ratings yet
- Chapter-5 Results: Department of Pharmaceutics, Mallige College of Pharmacy, BangaloreDocument55 pagesChapter-5 Results: Department of Pharmaceutics, Mallige College of Pharmacy, BangaloreSunil ChaudharyNo ratings yet
- Chapter-5 Results PDFDocument61 pagesChapter-5 Results PDFSunil ChaudharyNo ratings yet
- Bibliography of Fast Dissolving Tablet FormulationsDocument6 pagesBibliography of Fast Dissolving Tablet FormulationsSunil ChaudharyNo ratings yet
- Montelukast Sodium FDTs with Enhanced BioavailabilityDocument2 pagesMontelukast Sodium FDTs with Enhanced BioavailabilitySunil ChaudharyNo ratings yet
- Chapter-4 Materials and Methods PDFDocument16 pagesChapter-4 Materials and Methods PDFSunil ChaudharyNo ratings yet
- Review of Literature on Fast Dissolving TabletsDocument22 pagesReview of Literature on Fast Dissolving TabletsSunil ChaudharyNo ratings yet
- Content, List of Table, Figure and GraphsDocument6 pagesContent, List of Table, Figure and GraphsSunil ChaudharyNo ratings yet
- Fast dissolving tablets of Montelukast SodiumDocument2 pagesFast dissolving tablets of Montelukast SodiumSunil ChaudharyNo ratings yet
- Chapter-4 Materials and MethodsDocument15 pagesChapter-4 Materials and MethodsSunil ChaudharyNo ratings yet
- Fast dissolving tablets of Montelukast SodiumDocument2 pagesFast dissolving tablets of Montelukast SodiumSunil ChaudharyNo ratings yet
- Fast Dissolving Tablets: An OverviewDocument7 pagesFast Dissolving Tablets: An OverviewSunil ChaudharyNo ratings yet
- Acknowledging Support and Guidance in ResearchDocument7 pagesAcknowledging Support and Guidance in ResearchSunil ChaudharyNo ratings yet
- Review of Literature on Fast Dissolving TabletsDocument22 pagesReview of Literature on Fast Dissolving TabletsSunil ChaudharyNo ratings yet
- Need of Work and Study Need For The Study:: Chapter-2 Aim and ObjectiveDocument2 pagesNeed of Work and Study Need For The Study:: Chapter-2 Aim and ObjectiveSunil ChaudharyNo ratings yet
- Bibliography of Fast Dissolving Tablet FormulationsDocument6 pagesBibliography of Fast Dissolving Tablet FormulationsSunil ChaudharyNo ratings yet
- Need of Work and Study Need For The Study:: Chapter-2 Aim and ObjectiveDocument2 pagesNeed of Work and Study Need For The Study:: Chapter-2 Aim and ObjectiveSunil ChaudharyNo ratings yet
- Acknowledging Support and Guidance in ResearchDocument7 pagesAcknowledging Support and Guidance in ResearchSunil ChaudharyNo ratings yet
- Montelukast Sodium FDTs with Enhanced BioavailabilityDocument2 pagesMontelukast Sodium FDTs with Enhanced BioavailabilitySunil ChaudharyNo ratings yet
- 1.title Front Page PDFDocument1 page1.title Front Page PDFSunil ChaudharyNo ratings yet
- Title Front PageDocument2 pagesTitle Front PageSunil ChaudharyNo ratings yet
- Content, List of Table, Figure and GraphsDocument6 pagesContent, List of Table, Figure and GraphsSunil ChaudharyNo ratings yet
- Fast Dissolving Tablets: An OverviewDocument7 pagesFast Dissolving Tablets: An OverviewSunil ChaudharyNo ratings yet
- 21st Century Science - Summer 2011 - The Universe Is CreativeDocument84 pages21st Century Science - Summer 2011 - The Universe Is CreativeMatthew EhretNo ratings yet
- Transcript Ken Robinson 2010 NewDocument34 pagesTranscript Ken Robinson 2010 NewCarmen Maria FluturaşNo ratings yet
- Fitness RX For Women - December 2013Document124 pagesFitness RX For Women - December 2013renrmrm100% (2)
- Engineering Services for Abadan Petrochemical Plant VCM Unloading Station Piping DesignDocument28 pagesEngineering Services for Abadan Petrochemical Plant VCM Unloading Station Piping DesignMehdi NouriNo ratings yet
- Akbh PSK (V), TRBH As Y: AdhimokṣADocument8 pagesAkbh PSK (V), TRBH As Y: AdhimokṣA张晓亮No ratings yet
- MayankDocument38 pagesMayankmayank13430No ratings yet
- Surat Kecil Untuk TuhanDocument9 pagesSurat Kecil Untuk TuhanAsgarPurnamaNo ratings yet
- PAForge D20 Weapons CompendiumDocument29 pagesPAForge D20 Weapons Compendiumdjbonefish100% (1)
- Melese Hotel ST ReportDocument74 pagesMelese Hotel ST ReportKidist MollaNo ratings yet
- Dog Cake Recipe For Dozer's Birthday! - RecipeTin EatsDocument36 pagesDog Cake Recipe For Dozer's Birthday! - RecipeTin EatsZyreen Kate CataquisNo ratings yet
- 2011 02 Huijben Spie Why Every Urea Plant Needs A Continuous NC Meter PDFDocument9 pages2011 02 Huijben Spie Why Every Urea Plant Needs A Continuous NC Meter PDFfawadintNo ratings yet
- Solvent Extraction: Please Submit Question 4 For MarkingDocument3 pagesSolvent Extraction: Please Submit Question 4 For MarkingThembi Matebula100% (1)
- PDM TempDocument2 pagesPDM Tempamit rajputNo ratings yet
- CH 2.2: Separable Equations: X F DX DyDocument9 pagesCH 2.2: Separable Equations: X F DX DyPFENo ratings yet
- Siemens 810dDocument13 pagesSiemens 810dAdo ŠehićNo ratings yet
- #10 VHB SGT-APT Design SummaryDocument2 pages#10 VHB SGT-APT Design SummarySenthil KumarNo ratings yet
- Science: Pure Substances Vs MixturesDocument33 pagesScience: Pure Substances Vs MixturesElle Ma Rie100% (1)
- Slovakia C1 TestDocument7 pagesSlovakia C1 TestĐăng LêNo ratings yet
- Exercitii AdjectivDocument3 pagesExercitii AdjectivFirma GSCNo ratings yet
- Bandari 2015 Exact SER Expressions of GFDM in Nakagami-M and Rician Fading ChannelsDocument6 pagesBandari 2015 Exact SER Expressions of GFDM in Nakagami-M and Rician Fading Channelssameer khan100% (1)
- Growth, Stagnation or Decline? Agfficulturalproductm'Iy in British IndiaDocument290 pagesGrowth, Stagnation or Decline? Agfficulturalproductm'Iy in British IndiaHarshadeep BiswasNo ratings yet
- MODULE-2-VETTECH325 (2)Document31 pagesMODULE-2-VETTECH325 (2)cejproiloNo ratings yet
- Discover Over 70 Player Classes and RacesDocument1 pageDiscover Over 70 Player Classes and RacesFred FrançaNo ratings yet
- Biologic License ApplicationDocument16 pagesBiologic License ApplicationJean Sandra PintoNo ratings yet
- Rate Constant Determination 2Document8 pagesRate Constant Determination 2Divya UpadhyayNo ratings yet
- Analog Layout Design (Industrial Training)Document10 pagesAnalog Layout Design (Industrial Training)Shivaksh SharmaNo ratings yet
- Roland Berger Hot Trends InconstructionDocument24 pagesRoland Berger Hot Trends InconstructionJavier ContrerasNo ratings yet
- November 2010 (v1) QP - Paper 3 CIE Biology A-Level PDFDocument12 pagesNovember 2010 (v1) QP - Paper 3 CIE Biology A-Level PDFWiji NingNo ratings yet
- Accepted Manuscript: Process Safety and Environment ProtectionDocument51 pagesAccepted Manuscript: Process Safety and Environment Protectionimran shaukatNo ratings yet
- Waste Management in Vienna. MA 48Document12 pagesWaste Management in Vienna. MA 484rtttt4ttt44No ratings yet