Professional Documents
Culture Documents
TF Unidad1.3
TF Unidad1.3
Uploaded by
luis encarnacionOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
TF Unidad1.3
TF Unidad1.3
Uploaded by
luis encarnacionCopyright:
Available Formats
UNIVERSIDAD NACIONAL DE LOJA
de la Energía, las Industrias Facultad y los Recursos Naturales No Renovables
INGENIERÍA EN ELECTROMECÁNICA
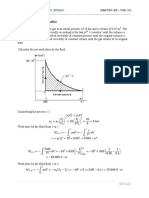
3. ¿Cuánto calor es necesario extraer a 0.2 kg de cada uno de los 3 primeros elementos
de la tabla 20.2 para convertirlos a estado sólido, si inicialmente están a temperatura
ambiente de 20°C? Calor específico Helio = 5193.2 J/(kg °C), Nitrógeno = 1040 J/(kg °C),
Oxígeno = 918 J/(kg °C).
Sol:
m ≔ 0.2 kg
Ti ≔ 20 °C
J
TfHf ≔ -269.65 °C TfHe ≔ -268.93 °C CpH ≔ 5193 ――
kg ⋅ K
J
TfNf ≔ -209.97 °C TfNe ≔ -195.81 °C CpN ≔ 1040 ――
kg ⋅ K
J
TfOf ≔ -218.79 °C TfOe ≔ -182.97 °C CpO ≔ 918 ――
kg ⋅ K
J J
LHf ≔ 5230 ― LHe ≔ 20900 ―
kg kg
J J
LNf ≔ 25500 ― LNe ≔ 201000 ―
kg kg
J J
LOf ≔ 13800 ― LOe ≔ 213000 ―
kg kg
QLv = m ⋅ Lv QLsol = m ⋅ Ls QV = -m ⋅ Cp ⋅ ∆T QS = -m ⋅ Cp ⋅ ∆T
QT = QLv + QLsol + QV + Qs
Helio
QTH ≔ m ⋅ LHf + m ⋅ LHe - m ⋅ CpH ⋅ ⎛⎝TfHe - Ti⎞⎠ - m ⋅ CpH ⋅ ⎛⎝TfHf - TfHe⎞⎠ = ⎛⎝3.061 ⋅ 10 5 ⎞⎠ J
Nitrogeno
QTN ≔ m ⋅ LNf + m ⋅ LNe - m ⋅ CpN ⋅ ⎛⎝TfNe - Ti⎞⎠ - m ⋅ CpN ⋅ ⎛⎝TfNf - TfNe⎞⎠ = ⎛⎝9.313 ⋅ 10 4 ⎞⎠ J
Oxigeno
QTO ≔ m ⋅ LOf + m ⋅ LOe - m ⋅ CpO ⋅ ⎛⎝TfOe - Ti⎞⎠ - m ⋅ CpO ⋅ ⎛⎝TfOf - TfOe⎞⎠ = ⎛⎝8.92 ⋅ 10 4 ⎞⎠ J
You might also like
- Assignment 4 TER1Y PDFDocument3 pagesAssignment 4 TER1Y PDFBogdan ŞipoşNo ratings yet
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Pipe Solved ProbsetDocument115 pagesPipe Solved ProbsetRemae Garci100% (1)
- TF Unidad1.1Document1 pageTF Unidad1.1luis encarnacionNo ratings yet
- Common ConstantsDocument3 pagesCommon Constantskristan7No ratings yet
- Fajanilan Jerome Exercise 3Document3 pagesFajanilan Jerome Exercise 3Jerome FajanilanNo ratings yet
- Solutions 4 F14Document5 pagesSolutions 4 F14nageshNo ratings yet
- 1 25 PDFDocument2 pages1 25 PDFJose IsaiasNo ratings yet
- CalculationsDocument2 pagesCalculationsLeoNo ratings yet
- Toaz - Info Refrigeration Moran Shapiro Solution Manual PRDocument10 pagesToaz - Info Refrigeration Moran Shapiro Solution Manual PREnhIe NkosiNo ratings yet
- Zeroth Law: Ifaandbandbandcarein Thermal Equil, Then A and C Are in Thermal EquilDocument52 pagesZeroth Law: Ifaandbandbandcarein Thermal Equil, Then A and C Are in Thermal Equilkamal El NasharNo ratings yet
- Cycles Revision SolutionsDocument11 pagesCycles Revision SolutionsLayla JhNo ratings yet
- Me 211 Examples SolutionsDocument30 pagesMe 211 Examples SolutionsBryan Dominic Gabriel PaduaNo ratings yet
- ch13 PDFDocument6 pagesch13 PDFAkash ThummarNo ratings yet
- Uygulama 1-2 & Ornek SorularDocument16 pagesUygulama 1-2 & Ornek SorularRSS RSSNo ratings yet
- Uygulama 1-2 & Ornek SorularDocument16 pagesUygulama 1-2 & Ornek SorularMuhittin SimsekNo ratings yet
- Thermodynamics 2 Quiz #3 - T01: Name: ID #: Problem:: 1 Mark 1 MarkDocument2 pagesThermodynamics 2 Quiz #3 - T01: Name: ID #: Problem:: 1 Mark 1 MarkPratulya KolheNo ratings yet
- Thermodynamic Processes: Processes of Ideal GasDocument3 pagesThermodynamic Processes: Processes of Ideal GasAngtiampo John AldrenNo ratings yet
- Sample Problem ThermoDocument25 pagesSample Problem ThermoJonnah Faye Mojares0% (1)
- ME 113 S09 HW2 SolutionDocument3 pagesME 113 S09 HW2 SolutionallyhawNo ratings yet
- Ideal Rankine CycleDocument20 pagesIdeal Rankine CycleJasmin TulosaNo ratings yet
- Me Ther - Act 5 Group2Document14 pagesMe Ther - Act 5 Group2ILAGAN ANNE FRANCINENo ratings yet
- Exercises Problem 1 Ref and Air Con MamaclayRADocument3 pagesExercises Problem 1 Ref and Air Con MamaclayRALeyzer MalumayNo ratings yet
- Property Calculations: Virial Equation of StateDocument9 pagesProperty Calculations: Virial Equation of Statesalman hussainNo ratings yet
- Sheet (1&2) ThermoDocument17 pagesSheet (1&2) ThermoAhmed A. TaimaNo ratings yet
- Thermo 5th Chap10 P001Document29 pagesThermo 5th Chap10 P001Rodrigo Andre Zuniga JuarezNo ratings yet
- Regenerative Rankine CycleDocument14 pagesRegenerative Rankine CycleSamson GabrielNo ratings yet
- Otto Cycle & Diesel CycleDocument36 pagesOtto Cycle & Diesel CycleNafisa AnikaNo ratings yet
- Chapter 10 Vapor and Combined Power CyclesDocument29 pagesChapter 10 Vapor and Combined Power Cyclesnamsun100% (1)
- Integrated Course 1 - Module 8 - Activity No. 2Document2 pagesIntegrated Course 1 - Module 8 - Activity No. 2aljon gonzalesNo ratings yet
- ThermodynamicallyDocument21 pagesThermodynamicallySunde PascuaNo ratings yet
- Gaspowercycle 150627103029 Lva1 App6892Document28 pagesGaspowercycle 150627103029 Lva1 App6892XADA YADANo ratings yet
- Applied Thermodynamics Exam 2018 Wirh SolutionsDocument9 pagesApplied Thermodynamics Exam 2018 Wirh SolutionsFarouk BassaNo ratings yet
- Ejercicio 2 MasaDocument7 pagesEjercicio 2 MasaCristian RafaelNo ratings yet
- Cartajena REVISEDDocument11 pagesCartajena REVISEDJerome Russel PublìcòNo ratings yet
- 12th PhysucsvipDocument3 pages12th Physucsvipphysics a2No ratings yet
- Chapter 5 Energy Balance April 10 2017.v2Document31 pagesChapter 5 Energy Balance April 10 2017.v2kennethmsorianoNo ratings yet
- Refrigeration Systems Quiz 1Document4 pagesRefrigeration Systems Quiz 1MartiNo ratings yet
- Thermodynamic & Transport Properties of Fluids (Rogers) 5eDocument13 pagesThermodynamic & Transport Properties of Fluids (Rogers) 5ewanameiNo ratings yet
- Perhitungan Neraca Massa: Tugas KhususDocument8 pagesPerhitungan Neraca Massa: Tugas KhususElmo LutchuuNo ratings yet
- Bilans It (New)Document1 pageBilans It (New)Ljubomir LukicNo ratings yet
- Energy Transfer by Heat, Work, and Mass: 1 Kpa M 1 K (N / M) M 1 KN M 1 KJDocument31 pagesEnergy Transfer by Heat, Work, and Mass: 1 Kpa M 1 K (N / M) M 1 KN M 1 KJAli Adel HassaniNo ratings yet
- Solved Board Problems - Gas Turbine Power PlantDocument8 pagesSolved Board Problems - Gas Turbine Power PlantFAMY Vazzim Soriano100% (1)
- ChE ThermodynamicsDocument49 pagesChE ThermodynamicsMiguel FelisildaNo ratings yet
- Quiz - 2: Group 2Document5 pagesQuiz - 2: Group 2ILAGAN ANNE FRANCINENo ratings yet
- At 2.5 Mpa 2803.1 6.2575 at 50 Kpa 340.49 1.0910 2305.4 6.2575 0.000103 Sol'N: 280.31Kj /KG Solving For H: + 6.2575 1.0910+X (6.5029)Document22 pagesAt 2.5 Mpa 2803.1 6.2575 at 50 Kpa 340.49 1.0910 2305.4 6.2575 0.000103 Sol'N: 280.31Kj /KG Solving For H: + 6.2575 1.0910+X (6.5029)Ariel Gamboa100% (1)
- Me ThermodynamicsDocument63 pagesMe ThermodynamicsGlenn Ray ErasmoNo ratings yet
- Thermodyancs Chapter 9 Solution ManuelDocument36 pagesThermodyancs Chapter 9 Solution ManuelFarhad MojaverNo ratings yet
- SARMIENTO Exercises Problem 2 Ref and Air ConDocument4 pagesSARMIENTO Exercises Problem 2 Ref and Air ConLeyzer MalumayNo ratings yet
- SARMIENTO Exercises Problem 2 Ref and Air ConDocument4 pagesSARMIENTO Exercises Problem 2 Ref and Air ConLeyzer MalumayNo ratings yet
- Module 3 Ideal Gases and Ideal Gas LawDocument12 pagesModule 3 Ideal Gases and Ideal Gas LawHazel AdoNo ratings yet
- Sample Calculation-BDocument14 pagesSample Calculation-BEdrielleNo ratings yet
- 4 Calorific Value CoalDocument2 pages4 Calorific Value Coalfarhan hyderNo ratings yet
- Chapter 3 (B) Energy Balance: 3.9 AssumptionsDocument21 pagesChapter 3 (B) Energy Balance: 3.9 Assumptionssaur1No ratings yet
- Boles Lecture Notes Thermodynamics Chapter 10Document15 pagesBoles Lecture Notes Thermodynamics Chapter 10prince assiriNo ratings yet
- Termodinámica 28Document12 pagesTermodinámica 28Ana LopezNo ratings yet
- Hfc134a (Kpa)Document33 pagesHfc134a (Kpa)Ayu LestariNo ratings yet
- May 6 Compressible-Flow-Homework Solutions: Mechanical Engineering 390 Fluid MechanicsDocument6 pagesMay 6 Compressible-Flow-Homework Solutions: Mechanical Engineering 390 Fluid MechanicsMuhammad UsmanNo ratings yet
- Question 1. During An Experiment Conducted in A Room at 25Document11 pagesQuestion 1. During An Experiment Conducted in A Room at 25fivos_rgNo ratings yet