Professional Documents
Culture Documents
Atomic Structure: Landmarks in The Evolution of Atomic Structure Are
Uploaded by
Mahmudul IslamOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Atomic Structure: Landmarks in The Evolution of Atomic Structure Are
Uploaded by
Mahmudul IslamCopyright:
Available Formats
CHEM1101: CHEMISTRY (EEE/COE) LECTURE 1
ATOMIC STRUCTURE
Landmarks in the evolution of atomic structure are:
1805 Dalton’s atomic theory
1896 Thomson discovery of electron and proton
1909 Rutherford’s nuclear atom
1913 Bohr’s atomic model
1921 Bohr-Bury scheme of electronic arrangement
1932 Chadwick’s discovery of the neutron
Atomic Theory of Dalton: (1805)
1. All matter is composed of tiny particles called atom which can not be created,
destroyed or splitted.
2. All atoms of any one element are identical, have same mass and chemical
properties.
3. A compound is a type of matter composed of atoms of two or more elements.
4. A chemical reaction consists of rearranging atoms from one combination to
another.
British Chemist John Dalton provided the basic theory: all matter- whether element,
compound, or mixture- is composed of small particles called atoms.
Limitations: (1) Atom can be divided into subatomic particle namely electron, proton and
neutron. (2) All atoms of any elements are not identical, have different mass and chemical
properties. This property is known as isotopes.
Rutherford’s Model of Atom: (1909)
1. Atom has a tiny dense center core or the NUCLEUS, which contains practically
the entire mass of the atom, leaving rest of the atom almost empty.
2. The entire positive charge of the atom is located on the nucleus, while electrons
were distributed in vacant space around it.
3. The electrons were moving in orbits or closed circular paths around the nucleus
like planets around the sun.
Rutherford's model of atom:
electrons orbiting around nucleus
Atomic Structure (Updated on Jan, 2014) Page 1
CHEM1101: CHEMISTRY (EEE/COE) LECTURE 1
Weakness of the Rutherford Atomic Model:
(1) Newton’s Laws of motion and gravitation can only be applied to neutral bodies such
as planets and not to charged bodies such as tiny electrons moving round a positive
nucleus. The analogy does not hold good since the electrons in an atom repel one
another, whereas planets attract each other because of gravitational forces. Besides,
there is electrostatic attraction in a nuclear atom model.
(2) According to Maxwell’s theory, and charged body such as electrons rotating in an
orbit must radiate energy continuously thereby losing kinetic energy. Hence the
electron must gradually spiral in towards the nucleus. The radius of the electron will
gradually decrease and it will ultimately fall into the nucleus, thus annihilating the
atom model.
(3) Since the process of radiating energy would go on continuously, the atomic spectra
should also be continuous and should not give sharp and well-defined lines.
Contribution: Rutherford laid the foundation of the model picture of atom.
Three subatomic particles or fundamental particles: Atoms can be subdivided into
smaller subatomic particles known as fundamental particles. Short description of three
subatomic particles is given below.
Mass Charge
Particle Symbol
amu grams Units Coulombs
Electron e- 1/1835 9.110-28 -1 -1.610-19
Proton p+ 1 1.67210-24 +1 +1.610-19
Neutron n or no 1 1.67410-24 0 0
Quantum Theory and Bohr Atom: (1913)
To understand the Bohr theory, we need to learn the nature of electromagnetic radiations
and atomic spectra.
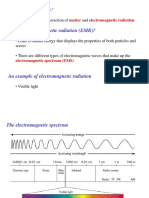
Electromagnetic Radiations:
Electromagnetic radiation can be described as a wave (carrier of energy) occurring
simultaneously in electrical and magnetic fields and consists of particles called quanta or
photons. Energy can be transmitted through space by electromagnetic radiations. Some forms
of electromagnetic radiations are - radio waves, visible light, infrared light, ultraviolet light,
X-rays etc.
These radiations have both the properties of a wave as well as a particle, now become
familiar for their uses. The X-rays are used in medicine, the ultraviolet rays lead to sunburns
and radio and radar waves used in communication and visible light.
Atomic Structure (Updated on Jan, 2014) Page 2
CHEM1101: CHEMISTRY (EEE/COE) LECTURE 1
Wave Length
Crest
Vibrating
Source Energy
Trough
Characteristics of Waves:
Wavelength (, lambda): The wavelength is defined as the distance between two successive
crests or troughs of a wave.
Units: cm, m or Å (angstrom).
1 Å = 10-8 cm = 10-10 m, 1 nm = 10-9 m
Frequency (, nu): The frequency is defined as the number of complete cycles (oscillations)
per second.
Units: hertz (hz), one cycle per second.
A wave of high frequency has a shorter wavelength, while a wave of low frequency has a
longer wavelength.
Speed: The speed (or velocity) of a wave is the distance through which a particular wave
travels in one second.
Speed = Frequency Wavelength (c = )
Wave Number: This is reciprocal of the wavelength and is given the symbol v (nu bar).
1
v =
Problem-1: The wavelength of a violet light is 400 nm. Calculate its frequency and wave
number. (c = 3 × 108m sec-1) (Answer: = 7.5 × 10-14 sec-1, v = 25 × 105 m-1)
Problem-2: The frequency of strong yellow line in the spectrum of sodium is 5.09 1014 sec-
1
. Calculate the wavelength of the light in nanometers. (Answer: = 589 nm)
Spectra: A spectrum is an arrangement of waves or particles spread out according to the
increasing or decreasing of some property like wavelength or frequency.
An increase in frequency or a decrease in wavelength represents an increase in energy.
Atomic Structure (Updated on Jan, 2014) Page 3
CHEM1101: CHEMISTRY (EEE/COE) LECTURE 1
Continuous Spectrum: White light is radiant energy coming from the sun or from
incandescent lamps. It is composed of light waves in the range 4000-8000 Å.
When a beam of white light is passed through a prism, a continuous series of colour bands
(rainbow: violet, indigo, blue, green, yellow, orange and red; VIBGYOR) is received on a
screen with different wavelengths called Continuous Spectrum.
The violet component of the spectrum has shorter wavelengths (4000-4250Å) and higher
frequencies.
The red component has longer wavelengths (6500-7500Å) and lower frequencies.
The invisible region beyond the violet is called ultraviolet region and the one below the red
is called infrared region.
ULTRAVIOLET
VIOLET o
4000 A
INDIGO
BLUE
PRISM GREEN
YELLOW
NARROW BEAM OF
ORANGE
WHITE LIGHT o
RED 6500 A
INFRARED
CONTINUOUS
SPECTRUM
Figure: The continuous spectrum of white light
Atomic Spectra:
When an element in the vapor or the gaseous state is heated in a flame or a discharge tube,
the atoms are excited and emit light radiations of a characteristic colour. The colour of light
produced indicates the wavelength of the radiation emitted. The spectrum obtained on the
photographic plate is found to consists of bright lines.
Na
3500 4000 4500 5000 5500 6000 6500 7000
Fig. Emission spectra of K, Na and H
Atomic Structure (Updated on Jan, 2014) Page 4
CHEM1101: CHEMISTRY (EEE/COE) LECTURE 1
Atomic Spectrum of Hydrogen: Balmer Equation and Rydberg constant
The emission line spectrum of hydrogen can be obtained by passing electric through the gas
contained in a discharge tube at low pressure. The light radiation emitted is then examined
with the help of a spectroscope.
In 1884 J. J. Balmer observed the following four prominent coloured lines in the visible
hydrogen spectrum:
(1) a red line with a wavelength of 6563 Å
(2) a blue-green line with a wavelength of 4861 Å
(3) a blue line with a wavelength of 4340 Å
(4) a violet line with a wavelength of 4102 Å
The above series of four lines in the visible spectrum of hydrogen is known as Balmer series.
PHOTOGRAPHIC
FILM
o
6563 A
o
HYDROGEN 4861 A
o
DISCHARGE TUBE 4340 A
GLASS PRISM
o
4102 A
LENS
SLIT
Figure: The examination of the atomic spectrum of hydrogen with a spectroscope.
Balmer was able to give an equation which relate the wavelengh () of the observed lines.
The Balmer equation is,
1 1 1
R 2 2
2 n
where R is a constant called Rydberg constant which has the value 109,677 cm-1 and n = 3,
4, 5, 6, etc.
Five spectral series: In addition to Balmer series, four other spectral series were discovered
in the infrared (ir) and ultraviolet (uv) regions of the hydrogen spectrum. These bear the
names of discoverers.
(1) Lyman series (uv)
(2) Balmer series (visible)
(3) Paschen series (ir)
(4) Brackett series (ir)
(5) Pfund series (ir)
Atomic Structure (Updated on Jan, 2014) Page 5
CHEM1101: CHEMISTRY (EEE/COE) LECTURE 1
Quantum Theory of Radiation:
(1) When atoms or molecules absorb or emit radiant energy, they do so in separate ‘units
of waves’ called quanta or photons.
Continuous
Wave
Photons or
quanta
individual photon
(2) The energy, E, of a quantum or photon is given by the relation.
E = h (h, Planck’s constant = 6.62 10–27 erg sec. Or 6.62 10-34 J sec.)
c = (c = velocity of radiation)
hc
Therefore, E =
(3) An atom or molecule can emit (or absorb) either one quantum of energy (h) or any
whole number multiple of this unit.
Bohr Model of the Atom:
(1) Electrons travel around the nucleus in specific permitted circular orbits and in no
others.
(2) While in these specific orbits, an electron does not radiate (or lose) energy.
(3) An electron can move from one energy level to another by quantum or photon
jumps only.
Electrons
Nucleus not allowed
between orbits
Electrons
permitted in
circular orbits 1
2
3
4
Orbit Nos
Atomic Structure (Updated on Jan, 2014) Page 6
CHEM1101: CHEMISTRY (EEE/COE) LECTURE 1
(4) The angular momentum (mvr) of an electron orbiting around the nucleus is an
integral multiple of Planck’s constant divided by 2.
nh
i.e. Angular momentum = mvr = ,
2
where m = mass of electron, v = velocity of electron, r = radius of the orbit, n = 1, 2,
3…..etc and h = Planck’s constant.
Calculation of Radius of Orbits:
Consider an electron of charge e revolving around a nucleus of charge Ze, where Z is the
atomic number and e the charge on a proton. Let m be the mass of the electron, r the radius of
the orbit and v the tangential velocity of the revolving electron.
The electrostatic force of attraction between the nucleus and the electron (Coulomb’s Law),
mv 2
r
Ze e
= Force of
r2 v e Attraction
The centrifugal force acting on the electron r
mv 2 Ze
=
r
Fig. Forces keeping
electron in orbit
Bohr assumed that these two opposing forces must be balancing each other exactly to keep
the electron in orbit. Thus,
Ze 2 mv 2
=
r2 r
For hydrogen Z=1, therefore,
e 2 mv 2
=
r2 r
Multiplying both sides by r
e2
= mv2 --- --- --- (1)
r
According to Bohr’s theory, angular momentum of the revolving electron is given by the
expression:
nh
mvr =
2
Atomic Structure (Updated on Jan, 2014) Page 7
CHEM1101: CHEMISTRY (EEE/COE) LECTURE 1
nh
or v = 2 mr --- --- --- (2)
Substituting the value of v in equation (1)
2
e2 nh
= m
2 mr
r
Solving for r,
n2h2
r --- --- --- (3)
4 2 me 2
Since the value of h, m and e had been determined experimentally, substituting these values
in (3), we have
8
r n 2 0.529 10 cm --- --- --- (4)
where n is the principal quantum number and hence the number of the orbit.
Problem-3: Calculate the first five Bohr radii of the hydrogen atom.
Problem-4: Calculate the radius of the third orbit of hydrogen atom.
Energy of Electron in each Orbit:
For hydrogen atom, the energy of the revolving electron, E is the sum of its kinetic energy
1 2 e2
mv and potential energy . (Hints: P.E.=kq1q2/r; attractive force, so negative)
2 r
1 2 e2
E = 2 mv --- --- --- (5)
r
From equation (1)
e2
mv 2
r
Substituting the value of mv2 in (5),
1 e2 e2
E= 2 r r
e2
E= --- --- --- (6)
2r
Substituting the value of r from equation (3) in (6),
e 2 4 2 me 2 2 2 me 4
E= = --- --- --- (7)
2 n2 h2 n2 h2
Substituting the values of m, e and h in (7),
Atomic Structure (Updated on Jan, 2014) Page 8
CHEM1101: CHEMISTRY (EEE/COE) LECTURE 1
2.179 10 11
E= erg / atom ,
n2
2.179 10 18
or E = J per atom --- --- --- (8)
n2
By using proper integer for n, we can get the energy for each orbit.
Problem-5: Calculate the five lowest energy levels of the hydrogen atom.
Problem-6: Calculate the energy of electron of the second orbit of the hydrogen atom.
Bohr Explanation of Hydrogen Spectrum:
The solitary electron in hydrogen atom at ordinary temperature resides in the first
orbit (n =1) and is in the lowest energy state (ground state).
When energy is supplied to hydrogen gas in the discharge tube, the electron moves to
higher energy levels viz., 2, 3, 4, 5, etc., depending on the quantity of energy
absorbed.
From these high energy levels, the electron returns by jumps to one or other lower
energy level.
In doing so the electron emits the excess energy as a photon.
This gives an excellent explanation of the various spectral series of hydrogen.
Lyman series is obtained when the electron returns to the ground state i.e., n = 1 from higher
levels (n2 = 2, 3, 4, 5, etc.). Similarly, Balmer, Paschen, Brackett and Pfund series are
produced when the electron returns to the second, third, fourth and fifth energy levels
respectively as shown in Figure.
7
Table: Spectral 6series of hydrogen
5
PFUND SERIES
Series n1 n2 Region Wavelength (Å)
4
Lyman 1 2, 3, 4, 5, etc. UltravioletBRACKETT920-1200
SERIES
Balmer 2 3, 4, 5, 6, etc. Visible 4000-6500
Paschen 33 4, 5, 6, 7, etc. InfraredSERIES
PASCHEN 9500-18750
Brackett 4 5, 6, 7 Infrared 19450-40500
Pfund 5 6, 7 Infrared 37800-75000
2
BALMER SERIES
ENERGY
LEVELS
Atomic Structure (Updated on Jan, 2014) GROUND STATE Page 9
1
LYMAN SERIES
FIGURE. Hydrogen spectral series on a Bohr atom energy diagram.
CHEM1101: CHEMISTRY (EEE/COE) LECTURE 1
Values of Rydberg’s constant is the same as in the original empirical Balmer’s equation
According to equation (7), the energy of the electron in orbit n1 (lower) and n2 (higher) is:
2 2 me4
E1 = 2
n1 h 2
2 2 me4
E2 = 2
n2 h 2
The difference of energy between the levels n1 and n2 is:
2 2 me 4 1 1
E E n2 E n1 2 2 --- --- --- (9)
h2 n1 n2
According to Planck’s equation
hc
E h --- --- --- (10)
where is wavelength of photon and c is velocity of light. From equation (9) and (10), we
can write
hc 2 2 me 4 1 1
= 2 2
h2 n1 n2
Atomic Structure (Updated on Jan, 2014) Page 10
CHEM1101: CHEMISTRY (EEE/COE) LECTURE 1
1 2 2 me 4 1 1
or, = 2
h 3 c n1 2 n2
1 1 1
or, = R 2 2 --- --- --- (11)
n1 n2
where R is Rydberg constant. The value of R can be calculated as the value of e, m, h and c
are known. It comes out to be 109,679 cm -1 and agrees closely with the value of Rydberg
constant in the original empirical Balmer’s equation (109,677 cm-1).
Calculation of wavelengths of the spectral lines of hydrogen in the visible region:
These lines constitute the Balmer series when n1 = 2. Now the equation (11) above can be
written as
1 1 1
= 109,679 2 2
2 n2
Thus the wavelengths of the photons emitted as the electron returns from energy levels 6, 5, 4
and 3 were calculated by Bohr. The calculated values corresponded exactly to the values of
wavelengths of the spectral lines already known. This was, in fact, a great success of the Bohr
atom.
Problem-7: Calculate the wavelength in Å of the line in Balmer series that is associated with
drop of the electron from the fourth orbit. The value of Rydberg constant is 109,676 cm-1.
Problem-8: Calculate the wavelength in Å of the third line in Balmer series that is associated
with drop of the electron. (Rydberg constant =109,676 cm-1).
Problem-9: The energy of the electron in the second and third orbits of the hydrogen atom is
-5.42 ×10-12 erg and -2.41×10-12 erg respectively. Calculate the wave length of the emitted
radiation when the electron drops from third to second orbit. (Answer: 6600Å)
Shortcoming of the Bohr Atom:
(1) It is unsuccessful for every other atom containing more than one electron,
(2) In view of modern advances, like dual nature of matter, uncertainty principle etc. any
mechanical model of the atom stands rejected.
(3) Bohr’s model of electronic structure could not account for the ability of atoms to form
molecules through chemical bonds.
(4) Bohr’s theory could not explain the effect of magnetic field (Zeeman effect) and
electric field (Stark effect) on the spectra of atoms.
Atomic Structure (Updated on Jan, 2014) Page 11
You might also like
- Atomic Structure: Chem1101: Chemistry (Eee/Coe)Document11 pagesAtomic Structure: Chem1101: Chemistry (Eee/Coe)Nabil AbdullahNo ratings yet
- Atomic StructureDocument11 pagesAtomic Structurereduan sadikNo ratings yet
- Atomic StructureDocument11 pagesAtomic StructureKH IFFTI HASANNo ratings yet
- 1.atom StructureDocument11 pages1.atom Structureroy2050No ratings yet
- Atomic Structure, Quantum Theory AND Bohr AtomDocument44 pagesAtomic Structure, Quantum Theory AND Bohr AtomMahmudul IslamNo ratings yet
- UV - Vis Spectros PDFDocument25 pagesUV - Vis Spectros PDFNelson BarriosNo ratings yet
- 9 The Electronic Structure of AtomsDocument16 pages9 The Electronic Structure of AtomsMr humanNo ratings yet
- Atomic Structure: Discovery of ElectronDocument9 pagesAtomic Structure: Discovery of ElectronHarsh GargNo ratings yet
- Structure of Atom: Chapter - 2Document13 pagesStructure of Atom: Chapter - 2Mukul MathurNo ratings yet
- SP01 - Atomic Theory To Bohr - PresentationDocument29 pagesSP01 - Atomic Theory To Bohr - PresentationMarianNo ratings yet
- Atomic Theory 3 ShareDocument31 pagesAtomic Theory 3 ShareRandom HoovyNo ratings yet
- Electron Configurations- Ionization EnergiesDocument33 pagesElectron Configurations- Ionization EnergiesRayan BotanyNo ratings yet
- Theoryofirspectroscopy 160622080111Document50 pagesTheoryofirspectroscopy 160622080111ArshNo ratings yet
- Electronic StructureDocument26 pagesElectronic StructureDavidson ChanNo ratings yet
- TDMU LightingEngineering Lecture1 M1.1 Light and RadiationDocument50 pagesTDMU LightingEngineering Lecture1 M1.1 Light and RadiationNguyễn ThịnhNo ratings yet
- Notes On Atomic Structure-1Document9 pagesNotes On Atomic Structure-1Manish AgrawalNo ratings yet
- Report SpectrosDocument4 pagesReport SpectrosFff FffNo ratings yet
- Atomic Structure and Models ExplainedDocument17 pagesAtomic Structure and Models ExplainedprashanthNo ratings yet
- Atomic Structure and Quantum TheoryDocument21 pagesAtomic Structure and Quantum TheoryprishaNo ratings yet
- Atomic Structure JEE Main and Advanced (Theory)Document19 pagesAtomic Structure JEE Main and Advanced (Theory)Er. Vineet Loomba (IIT Roorkee)No ratings yet
- Atomic Theory Notes 2019 Answers PDFDocument16 pagesAtomic Theory Notes 2019 Answers PDFJohnNo ratings yet
- UV - Visible SpectrophotometryDocument171 pagesUV - Visible SpectrophotometryGudisa KufoNo ratings yet
- Chapter 2.1 - Structure of AtomDocument46 pagesChapter 2.1 - Structure of AtomHakim Abbas Ali PhalasiyaNo ratings yet
- Share '1-Photelectric EffectDocument43 pagesShare '1-Photelectric EffectẄâQâŗÂlïNo ratings yet
- Chapter14 Atomic StructureDocument10 pagesChapter14 Atomic StructureDebasish BagNo ratings yet
- Instrumental Analysis Spectroscopy - ppt12Document109 pagesInstrumental Analysis Spectroscopy - ppt12kiya01No ratings yet
- CHEM 205 Chapter 6Document29 pagesCHEM 205 Chapter 6phikjaeNo ratings yet
- Atomic Structure PDFDocument28 pagesAtomic Structure PDFArun Kumar100% (2)
- Structure and Spectra of Hydrogenic AtomsDocument28 pagesStructure and Spectra of Hydrogenic AtomssernaNo ratings yet
- Chapter Two. EEE & ETI 1204 Material ScienceDocument29 pagesChapter Two. EEE & ETI 1204 Material ScienceKIRAGU PURITY NJERINo ratings yet
- VIP Nuclear BasicsDocument166 pagesVIP Nuclear BasicsgetachewNo ratings yet
- Unit-1 (B - MARCH 2022Document40 pagesUnit-1 (B - MARCH 2022Vibin SimonNo ratings yet
- Infrared Spectroscopy ExplainedDocument42 pagesInfrared Spectroscopy ExplainedMade PrimaNo ratings yet
- Modern Physics Hints: Atomic Structure, Spectra, Radiation, and MoreDocument21 pagesModern Physics Hints: Atomic Structure, Spectra, Radiation, and MoreOlajide HeritageNo ratings yet
- Presentation of UV-Visible SpectrophotometrDocument34 pagesPresentation of UV-Visible SpectrophotometranuvasNo ratings yet
- Notes Radiation Teacher - 2017Document10 pagesNotes Radiation Teacher - 2017smedificationNo ratings yet
- CBSE Class 11 Chemistry Notes: Atomic StructureDocument13 pagesCBSE Class 11 Chemistry Notes: Atomic StructureSonaakshi Jagadeesh BabuNo ratings yet
- Chemistry: UNIT 1 - Module 1 Atomic StructureDocument36 pagesChemistry: UNIT 1 - Module 1 Atomic StructureAntonique HeadmanNo ratings yet
- Chapter 2.1 - Structure of Atom 2Document60 pagesChapter 2.1 - Structure of Atom 2Hakim Abbas Ali PhalasiyaNo ratings yet
- Chem U 2 G 11Document23 pagesChem U 2 G 11EApp And AgreementNo ratings yet
- 11 Chemistry Notes Ch02 Structure of AtomDocument18 pages11 Chemistry Notes Ch02 Structure of AtomSayantanBanerjee0% (1)
- Lect2-Light ReactionDocument62 pagesLect2-Light ReactionGilang Satya ArdhanaNo ratings yet
- Chem 442 - Chapter OneDocument26 pagesChem 442 - Chapter OneArindam DasNo ratings yet
- L3-4. UV SpectrosDocument33 pagesL3-4. UV Spectrosngocnm.bi12-320No ratings yet
- Chapter No 5 Work SheetDocument10 pagesChapter No 5 Work SheetNabeel RazaNo ratings yet
- UNESCO-NIGERIA TECHNICAL & VOCATIONAL EDUCATION REVITALISATION PROJECT-PHASE II NATIONAL DIPLOMA IN SCIENCE LABORATORY TECHNOLOGY GENERAL PRINCIPLES OF CHEMISTRYDocument65 pagesUNESCO-NIGERIA TECHNICAL & VOCATIONAL EDUCATION REVITALISATION PROJECT-PHASE II NATIONAL DIPLOMA IN SCIENCE LABORATORY TECHNOLOGY GENERAL PRINCIPLES OF CHEMISTRYTosin AbiolaNo ratings yet
- Atom and Subatomic Particles ExplainedDocument16 pagesAtom and Subatomic Particles ExplainedMahin ChandwaniNo ratings yet
- Electron ConfigurationsDocument15 pagesElectron ConfigurationsEdwineNo ratings yet
- Phy 104 Atomic SpectraDocument24 pagesPhy 104 Atomic SpectraAisha abba HabibNo ratings yet
- Uv-Spectroscopy Handout by Vijaya Lakshmi Sada - pdf524Document13 pagesUv-Spectroscopy Handout by Vijaya Lakshmi Sada - pdf524manasjyotiray02186No ratings yet
- Structure - of - Atom - Class 11 CbseDocument10 pagesStructure - of - Atom - Class 11 CbseAnshuman SinghNo ratings yet
- Chap 12 - AtomsDocument7 pagesChap 12 - AtomsLOVKUSH PANDEYNo ratings yet
- Chap 12 - AtomsDocument7 pagesChap 12 - AtomsArpan KumarNo ratings yet
- Chap 12 - AtomsDocument7 pagesChap 12 - AtomsSaksham KhandelwalNo ratings yet
- XRF Analysis of Elemental CompositionDocument14 pagesXRF Analysis of Elemental CompositionGandis YulianaNo ratings yet
- Elementary Particles: The Commonwealth and International LibraryFrom EverandElementary Particles: The Commonwealth and International LibraryNo ratings yet
- Optical Sources, Detectors, and Systems: Fundamentals and ApplicationsFrom EverandOptical Sources, Detectors, and Systems: Fundamentals and ApplicationsNo ratings yet
- Fundamentals of Energy Dispersive X-Ray Analysis: Butterworths Monographs in MaterialsFrom EverandFundamentals of Energy Dispersive X-Ray Analysis: Butterworths Monographs in MaterialsRating: 5 out of 5 stars5/5 (1)
- Liban AnalyseDocument12 pagesLiban AnalyseSatish ReddyNo ratings yet
- Processed oDocument2 pagesProcessed oHemanth KumarNo ratings yet
- Milking MachineDocument10 pagesMilking Machineuniversaldairy100% (2)
- Bankruptcy Judge Imposes Sanctions On CounselDocument17 pagesBankruptcy Judge Imposes Sanctions On Counsel83jjmackNo ratings yet
- Read Me 22222222222222Document2 pagesRead Me 22222222222222sancakemreNo ratings yet
- Cookery 10Document5 pagesCookery 10Angelica CunananNo ratings yet
- Sheet-Pan Salmon and Broccoli With Sesame and Ginger: by Lidey HeuckDocument2 pagesSheet-Pan Salmon and Broccoli With Sesame and Ginger: by Lidey HeuckllawNo ratings yet
- 2.visual ArtsDocument78 pages2.visual ArtsNelson VergaraNo ratings yet
- Exam On Multiculturalism: - Acculturation Process of Immigrants From Central and Eastern Europe in SwedenDocument60 pagesExam On Multiculturalism: - Acculturation Process of Immigrants From Central and Eastern Europe in SwedenMarta santosNo ratings yet
- DSE2541 Data SheetDocument2 pagesDSE2541 Data SheetBERANGER DAVESNE DJOMALIA SIEWENo ratings yet
- Is College For Everyone - RevisedDocument5 pagesIs College For Everyone - Revisedapi-295480043No ratings yet
- SWOT ANALYSIS - Docx Sime DarbyDocument9 pagesSWOT ANALYSIS - Docx Sime Darbynur azfarinie ruzliNo ratings yet
- Cyber Crime and Law ClassDocument99 pagesCyber Crime and Law ClassMohd ShifanNo ratings yet
- Conciliar Post Reference GuideDocument9 pagesConciliar Post Reference GuideBenjamin WinterNo ratings yet
- Operon NotesDocument3 pagesOperon NotesanithagsNo ratings yet
- Handbook+of+Global+Tuberculosis+Control - Chapter 5 - 2Document17 pagesHandbook+of+Global+Tuberculosis+Control - Chapter 5 - 2Dyah MustikawatiNo ratings yet
- Flamme Rouge - Grand Tour Rules - English-print-V2Document3 pagesFlamme Rouge - Grand Tour Rules - English-print-V2dATCHNo ratings yet
- Thời gian làm bài: 90 phút (không kể thời gian giao đề)Document8 pagesThời gian làm bài: 90 phút (không kể thời gian giao đề)Nguyễn KiênNo ratings yet
- Explosive Ordnance Disposal & Canine Group Regional Explosive Ordnance Disposal and Canine Unit 3Document1 pageExplosive Ordnance Disposal & Canine Group Regional Explosive Ordnance Disposal and Canine Unit 3regional eodk9 unit3No ratings yet
- UNIX Unbounded 5 Edition: Amir AfzalDocument23 pagesUNIX Unbounded 5 Edition: Amir AfzalOsei BanningNo ratings yet
- 7 Tips For A Tidy Desk - ExercisesDocument4 pages7 Tips For A Tidy Desk - Exercisesjosh acNo ratings yet
- Song Activity by Christina AguileraDocument1 pageSong Activity by Christina AguileraRossita BasharovaNo ratings yet
- An Umbrella For Druvi: Author: Shabnam Minwalla Illustrator: Malvika TewariDocument12 pagesAn Umbrella For Druvi: Author: Shabnam Minwalla Illustrator: Malvika TewariKiran Kumar AkulaNo ratings yet
- Tetative Teaching Plan of Intro To BusinessDocument5 pagesTetative Teaching Plan of Intro To BusinessAhmed RaXaNo ratings yet
- Question 1 - Adjusting EntriesDocument10 pagesQuestion 1 - Adjusting EntriesVyish VyishuNo ratings yet
- Economics I Course Manual 2022Document13 pagesEconomics I Course Manual 2022Mridula BansalNo ratings yet
- Insertion Mangement Peripheral IVCannulaDocument20 pagesInsertion Mangement Peripheral IVCannulaAadil AadilNo ratings yet
- Salah or Prayer Is One of The Pillars of Islam and It Is AnDocument7 pagesSalah or Prayer Is One of The Pillars of Islam and It Is AnSitti Nauhar AukasaNo ratings yet
- Explore the City Centre in Salt Lake, KolkataDocument7 pagesExplore the City Centre in Salt Lake, Kolkataaishwarya raniNo ratings yet
- q2 Wk1 Worksheet1 Music8Document10 pagesq2 Wk1 Worksheet1 Music8Michie Maniego - GumanganNo ratings yet