Professional Documents
Culture Documents
Adiabatic Process and Polytropic Process
Uploaded by
Avinash GarikapatiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Adiabatic Process and Polytropic Process
Uploaded by
Avinash GarikapatiCopyright:
Available Formats
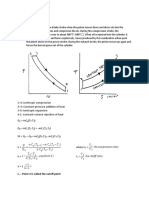
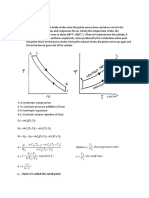
Adiabatic process
An adiabatic process is a process in which no heat transfer takes place. As no heat transfer takes
place into or out of the system it is an isentropic process. This process is of the form:
Pvk = constant --------------------------------------------------------------------------(1)
where,
P = pressure,
v = volume,
k = ratio of specific heats (Cp/Cv)
Polytropic process
A polytropic process is a variable entropy process in which heat transfer takes place during the
process. It is of the form:
Pvn = constant --------------------------------------------------------------------------- (2)
where,
n = k for an adiabatic, isentropic process
= 1 for an isothermal process
= 0 for an isobaric process
= ∞ for an isochoric process
From equation (2) we may write for a polytropic process:
P1v1n = P2v2n = constant
where,
P1 = initial pressure,
P2 = final pressure,
v1 = initial volume,
v2 = final volume,
P2 / P1 = (v1/v2)n ------------------------------------------------------------------------ (3)
Also,
P1v1/T1 = P2v2/T2
v1/v2 = P2T1/P1T2 ----------------------------------------------------------------------- (4)
Using (4) in (3):
P2/P1 = (P2T1/P1T2)n
(P2/P1)(1-n) = (T2/T1)-n
Defining P2/P1 = rP and T2/T1 as rT we can write:
rT = rP(n-1)/n -------------------------------------------------------------------------------- (5)
Taking logarithm on both sides:
ln rT = (n-1)/n ln rP
n/(n-1) = (ln rP)/( ln rT) ----------------------------------------------------------------(6)
Polytropic head and Polytropic efficiency
Polytropic head is defined as the work output per mass of fluid. For a steady state, steady flow
process, the polytropic head is given by the following relationship:
HP = 1∫2 vdP ----------------------------------------------------------------------------- (7)
From equation (2):
Pvn = constant = Cn --------------------------------------------------------------------- (8)
v = C/P1/n
Substituting v in (7) we have:
HP = 1∫2 C/P1/n dP
= C/(1-1/n)*P1-(1/n) 1│ 2
= CnP(n-1)/n/(n-1) 1│ 2
= Cn/(n-1) * [P2(n-1)n – P1(n-1)n]
= Cn P1(n-1)n/(n-1)*[(P2/P1)(n-1)/n - 1]
= Cn P1(n-1)n/(n-1)*[rP(n-1)/n - 1] --------------------------------------------------- (9)
From (8) we have:
Pvn = Cn
C = (Pvn)1/n = P11/n v1
Using the value of C in (9):
HP = nP11/n v1 P1(n-1)n/(n-1)*[rP(n-1)/n - 1]
= n/(n-1) * P1v1[rP(n-1)/n - 1] ------------------------------------------------------ (10)
From the ideal gas law:
P1v1 = RT1
where,
v = specific volume
R = gas constant = function (molecular weight)
= 8314/mol.wt
. Nm/kgK in metric system
Using this in equation (10) we have:
HP = n/(n-1) * RT1[rP(n-1)/n - 1] ----------------------------------------------------- (11)
Polytropic efficiency of a compression process is defined as the ratio of the work output to the
work input.
The work input per mass of fluid is the change of enthalpy of the gas.
w = ∆h
= cp(T2 - T1)
= cp T1(T2/ T1 - 1)
= cp T1(rT - 1)
Using (5) in the above equation:
w = cp T1(rP(n-1)/n - 1) ----------------------------------------------------------------- (12)
But,
cp = Rk/(k - 1)
Using this in (12):
w = Rk/(k - 1) * T1(rP(n-1)/n - 1) ------------------------------------------------------ (13)
Therefore, polytropic efficiency,
ηP = Hp/w
n /(n−1)∗RT 1 [r P(n−1)/n−1]
=
k /( k−1)∗RT 1(r P(n−1)/n−1)
n/( n−1)
ηP = ----------------------------------------------------------------------------(14)
k /( k−1)
Dependence of polytropic efficiency on inlet and outlet conditions.
Combining equation (6) and (14) and assuming ‘k’ to remain constant under all circumstances,
we can say:
ηP α (ln rP)/( ln rT)
Therefore, the polytropic efficiency of a stage of a multi-stage centrifugal compressor is directly
proportional to the pressure ratio and inversely proportional to the temperature ratio of the given
stage. Thus, for a process with constant inlet pressure and temperature condition, best
polytropic efficiency will be achieved if maximum pressure rise can be achieved for a
minimum possible temperature rise.
You might also like
- CH 02Document20 pagesCH 02Pauline Nguyen100% (1)
- Exercises in Electronics: Operational Amplifier CircuitsFrom EverandExercises in Electronics: Operational Amplifier CircuitsRating: 3 out of 5 stars3/5 (1)
- Process Calculations 2nd Ed. - V. Venkataramani, N. Anantharaman & K.M. Meera Sheriffa Begum 2011 PDFDocument260 pagesProcess Calculations 2nd Ed. - V. Venkataramani, N. Anantharaman & K.M. Meera Sheriffa Begum 2011 PDFantonino69100% (6)
- Solutions, and The Optional Wickert Textbook Only. No Electronic Devices Other Than Calculators Will BeDocument4 pagesSolutions, and The Optional Wickert Textbook Only. No Electronic Devices Other Than Calculators Will BenuncafalhaNo ratings yet
- Dual CycleDocument3 pagesDual CycleMohammad Faraz AkhterNo ratings yet
- Thermodynamics 2 E7Document41 pagesThermodynamics 2 E7taya699No ratings yet
- Reciprocal CompressorsDocument40 pagesReciprocal CompressorsSathish KasilingamNo ratings yet
- Physics FormulasDocument6 pagesPhysics FormulasRam PrasadNo ratings yet
- Non Flow Processes Gases Closed SystemDocument9 pagesNon Flow Processes Gases Closed SystemNjabulo NgobeseNo ratings yet
- Unit: 4Document25 pagesUnit: 4Vikas MishraNo ratings yet
- Air Compressor1Document24 pagesAir Compressor1Sayem kaifNo ratings yet
- Unit: 4Document27 pagesUnit: 4Vikas MishraNo ratings yet
- Peng-Robinson Fortran ProgramDocument12 pagesPeng-Robinson Fortran ProgramSamuel Pangeran SilalahiNo ratings yet
- Axial CompressorDocument18 pagesAxial CompressorSumiran ManghaniNo ratings yet
- Mover, Otherwise Known As The Turbine. For: R P S T K XDocument16 pagesMover, Otherwise Known As The Turbine. For: R P S T K XMohamad SannanNo ratings yet
- TD Unit 2Document6 pagesTD Unit 2Shine SonNo ratings yet
- DatasheetDocument16 pagesDatasheetSandu VisanescuNo ratings yet
- Formula Overview (Aeronautics)Document8 pagesFormula Overview (Aeronautics)Aishwarya RaviNo ratings yet
- Isochronal Test3Document36 pagesIsochronal Test3Veviet pomataNo ratings yet
- POWER FACTOR CORRECTION For DUMMIESDocument1 pagePOWER FACTOR CORRECTION For DUMMIESJin WuNo ratings yet
- CRE SUBJECT DoubtClearnaceDocument2 pagesCRE SUBJECT DoubtClearnaceDhanu DhanushNo ratings yet
- Gas Power Cycles Study Guide in Powerpoint: To AccompanyDocument68 pagesGas Power Cycles Study Guide in Powerpoint: To AccompanyManjunatha TnNo ratings yet
- Gas Reservoirs6Document35 pagesGas Reservoirs6Veviet pomataNo ratings yet
- Ic Engine Cycles 1Document85 pagesIc Engine Cycles 1jhpandiNo ratings yet
- ThermodynamicsNotes PDFDocument41 pagesThermodynamicsNotes PDFAsia CtNo ratings yet
- Lte KomutlarDocument6 pagesLte Komutlaremre kayaNo ratings yet
- Prelim Ito Na PreDocument8 pagesPrelim Ito Na PreJethro Briza GaneloNo ratings yet
- Command 2G 3G 4G 18052022Document66 pagesCommand 2G 3G 4G 18052022yacine bouazniNo ratings yet
- Timing Diagram Timers CycleDocument1 pageTiming Diagram Timers Cycledon121don121No ratings yet
- Nota PemampatDocument49 pagesNota PemampatweafareezNo ratings yet
- Chap3 4Document10 pagesChap3 4rpb109100% (1)
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocument7 pagesDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNo ratings yet
- Lecture Notes OnDocument200 pagesLecture Notes Onananth k r100% (3)
- Math Formula ListDocument3 pagesMath Formula ListKumaravel PadmaroopaNo ratings yet
- Thermodynamics FormulasDocument16 pagesThermodynamics FormulasMohammad SaleemNo ratings yet
- For An Isentropic Process S SDocument11 pagesFor An Isentropic Process S SRoshan ShanmughanNo ratings yet
- Otto CycleDocument28 pagesOtto CycleNazeeh Abdulrhman AlbokaryNo ratings yet
- Thermodynamic CyclesDocument30 pagesThermodynamic CyclesRudra PratapNo ratings yet
- MmcinfinityDocument17 pagesMmcinfinityKartik GuptaNo ratings yet
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocument8 pagesDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNo ratings yet
- Ambiga JDocument120 pagesAmbiga Jela666666No ratings yet
- ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ − − = T T 1 T T 1 1 η: www.freestudy.co.ukDocument1 page⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ − − = T T 1 T T 1 1 η: www.freestudy.co.ukcataiceNo ratings yet
- Power System Simulation Laboratory: A RecordDocument120 pagesPower System Simulation Laboratory: A Recordela666666No ratings yet
- Proracun Gasne TurbineDocument15 pagesProracun Gasne TurbineToni TalevskiNo ratings yet
- Patterns While Loop Part-1Document13 pagesPatterns While Loop Part-1vsnpradeepNo ratings yet
- P P A U M T A M P P U U: AS301, IITM, Lecture 2 (06/08/2007)Document3 pagesP P A U M T A M P P U U: AS301, IITM, Lecture 2 (06/08/2007)Sunil VermaNo ratings yet
- Module 33D - Closed Loop Proportional-Integral Pressure ControlDocument4 pagesModule 33D - Closed Loop Proportional-Integral Pressure ControlrhedmishNo ratings yet
- Formulario para Ser Utilizado Durante El Examen TransformadoresDocument1 pageFormulario para Ser Utilizado Durante El Examen TransformadoresFernando Manuel Fernandez GhisoNo ratings yet
- System Design Assignment - 1: SUBMITTED BY:-P.D.ANEESH (113079017) VISMAY DESAI (11307R007)Document10 pagesSystem Design Assignment - 1: SUBMITTED BY:-P.D.ANEESH (113079017) VISMAY DESAI (11307R007)PD AneeshNo ratings yet
- Assignment Exp 5ADocument6 pagesAssignment Exp 5AMohammed TAOUSSINo ratings yet
- Propeller Design Detail StageDocument4 pagesPropeller Design Detail Stageleokyawlay87No ratings yet
- Fa17-Eee-008 - Asssignment 4Document6 pagesFa17-Eee-008 - Asssignment 4Hammad SattiNo ratings yet
- Thus, This Term Actually Means A in A Constant-Volume System The Measure of Reaction Rate of Component I BecomesDocument23 pagesThus, This Term Actually Means A in A Constant-Volume System The Measure of Reaction Rate of Component I Becomesalice Annabelle100% (1)
- Engineering Software: Otto Cycle AnalysisDocument14 pagesEngineering Software: Otto Cycle AnalysisAnonymous J6blfqNo ratings yet
- Ee 705-Vlsi Design Lab: Describing A Finite State Machine (FSM)Document10 pagesEe 705-Vlsi Design Lab: Describing A Finite State Machine (FSM)Indranil ChakrabortyNo ratings yet
- Op Amp ApplicationDocument15 pagesOp Amp ApplicationChris Real PabiaNo ratings yet
- TutUM UM MarcH-2017Document6 pagesTutUM UM MarcH-2017Dennis LingNo ratings yet
- Plant Engineering 2 - Compressors 1 The Perfect Gas ModelDocument12 pagesPlant Engineering 2 - Compressors 1 The Perfect Gas ModelDee RajanNo ratings yet
- PNEUMATICS AND AIR CIRCUITS UNDERSTANDING THE CASCADE VALVE AND PLC UNDERSTANDINGFrom EverandPNEUMATICS AND AIR CIRCUITS UNDERSTANDING THE CASCADE VALVE AND PLC UNDERSTANDINGNo ratings yet
- Reference Guide To Useful Electronic Circuits And Circuit Design Techniques - Part 2From EverandReference Guide To Useful Electronic Circuits And Circuit Design Techniques - Part 2No ratings yet
- Pump Classifications:: Transportation of FluidsDocument2 pagesPump Classifications:: Transportation of FluidsAvinash GarikapatiNo ratings yet
- Adiabatic ProcessDocument1 pageAdiabatic ProcessAvinash GarikapatiNo ratings yet
- Page NoDocument3 pagesPage NoAvinash GarikapatiNo ratings yet
- Chemical CompaniesDocument3 pagesChemical CompaniesAvinash GarikapatiNo ratings yet
- Fluid Mechanics IntroductionDocument4 pagesFluid Mechanics IntroductionAvinash GarikapatiNo ratings yet
- Compressible and Incompressible FluidDocument3 pagesCompressible and Incompressible FluidAvinash GarikapatiNo ratings yet
- Extraction of Essential OilsDocument9 pagesExtraction of Essential OilsAvinash GarikapatiNo ratings yet
- ExtractionDocument13 pagesExtractionAvinash GarikapatiNo ratings yet
- Purchasing Function ImplementationDocument2 pagesPurchasing Function ImplementationAvinash GarikapatiNo ratings yet
- Purchasing Function ImplementationDocument2 pagesPurchasing Function ImplementationAvinash GarikapatiNo ratings yet
- Purchasing Function ImplementationDocument2 pagesPurchasing Function ImplementationAvinash GarikapatiNo ratings yet
- Unsteady State Batch ReactorDocument11 pagesUnsteady State Batch ReactorAvinash GarikapatiNo ratings yet
- Extraction of Essential OilsDocument9 pagesExtraction of Essential OilsAvinash GarikapatiNo ratings yet
- Tin ExtractionDocument10 pagesTin ExtractionAvinash GarikapatiNo ratings yet
- Pms Mini ProjectDocument7 pagesPms Mini ProjectAvinash GarikapatiNo ratings yet
- FiltarationDocument22 pagesFiltarationAvinash GarikapatiNo ratings yet
- Binomial DistributionDocument10 pagesBinomial DistributionAvinash GarikapatiNo ratings yet
- Photoelectric PyrometerDocument13 pagesPhotoelectric PyrometerAvinash Garikapati100% (1)
- Entropy ChangeDocument13 pagesEntropy Changeنبيل محمد عيد ابوميراNo ratings yet
- Fenwick & Deam - Design of Opening Corners Between Reinforced Concrete Walls and Slabs Vol 16 No 1 2003Document7 pagesFenwick & Deam - Design of Opening Corners Between Reinforced Concrete Walls and Slabs Vol 16 No 1 2003Ivy HuaNo ratings yet
- 1.pre test-GEN-ENGDocument4 pages1.pre test-GEN-ENGWilson AtienzaNo ratings yet
- Practical Rotordynamics For Centrifugal PumpsDocument52 pagesPractical Rotordynamics For Centrifugal PumpsEric21No ratings yet
- SL 316 - Machine Design - I - Sem V - May 2017Document7 pagesSL 316 - Machine Design - I - Sem V - May 2017Anantkumar GujarNo ratings yet
- Laminar Flow Aircraft Certification: NAS Publication 2413Document338 pagesLaminar Flow Aircraft Certification: NAS Publication 2413Olga Lucia Duque QuijanoNo ratings yet
- Physics Waec Syllabus 2023Document1 pagePhysics Waec Syllabus 2023fasehunrachealoluwaseunNo ratings yet
- Alexandria Higher Institute of Engineering & Technology 2020/2021Document4 pagesAlexandria Higher Institute of Engineering & Technology 2020/2021khaled mohamedNo ratings yet
- River Engineering and Sediment Transport Solved ProbelemDocument6 pagesRiver Engineering and Sediment Transport Solved ProbelemMaulid100% (12)
- Phy 1 23 Lab 4 - Centripetal ForceDocument3 pagesPhy 1 23 Lab 4 - Centripetal ForceGrace KimNo ratings yet
- Column Design Examples EBCSDocument7 pagesColumn Design Examples EBCSMesfin Derbew89% (28)
- Horizontal Directional DrillingDocument15 pagesHorizontal Directional Drillingoconnorr8133% (3)
- Installation and Extraction of Spudcans Using Abaqus/Explicit 2010Document1 pageInstallation and Extraction of Spudcans Using Abaqus/Explicit 2010SIMULIACorpNo ratings yet
- Design of HP HT Pipelines Against Lateral BucklingDocument20 pagesDesign of HP HT Pipelines Against Lateral BucklingHarmoni Andreas100% (1)
- Torque On A CoilDocument24 pagesTorque On A CoiledithNo ratings yet
- Fieldtheory Uniform Plane WavesDocument91 pagesFieldtheory Uniform Plane WavesedwinNo ratings yet
- Design of Slider CrankDocument3 pagesDesign of Slider CranketemecNo ratings yet
- Airfoil Tip Vortex Formation NoiseDocument7 pagesAirfoil Tip Vortex Formation NoiseSara RodriguezNo ratings yet
- Dynamics (Dinamika)Document38 pagesDynamics (Dinamika)bat.laughNo ratings yet
- Chaotic Dynamics of Repeated Impacts in Vibratory Bowl FeedersDocument13 pagesChaotic Dynamics of Repeated Impacts in Vibratory Bowl Feedersأحمد عاطف أبوغديرNo ratings yet
- 4-Lrfd Method W NotesDocument23 pages4-Lrfd Method W NotesNina Ysabelle RamosNo ratings yet
- NotesDocument3 pagesNotesS Sweet SweetNo ratings yet
- Motion of Charged Particles in Electric Fields W BlanksDocument4 pagesMotion of Charged Particles in Electric Fields W BlanksAshir50% (2)
- INDIAN Steel TableDocument6 pagesINDIAN Steel TableKingshuk SarkarNo ratings yet
- 1.answers To Physics Question BankDocument18 pages1.answers To Physics Question BankMuhammad YousafNo ratings yet
- Steel Connections Theory Enu PDFDocument120 pagesSteel Connections Theory Enu PDFTiago Castelani100% (1)
- Ocean Engineering and Naval Architecture: Vibration of Floating StructuresDocument14 pagesOcean Engineering and Naval Architecture: Vibration of Floating StructuresSuraj GaikwadNo ratings yet
- Eddy Current BrakesDocument24 pagesEddy Current BrakesMd Jawed AkhtarNo ratings yet