Professional Documents
Culture Documents
Repaglinide: Chemical Name: (S) - (
Uploaded by
Rhenso Victor Albites CondoriOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Repaglinide: Chemical Name: (S) - (
Uploaded by
Rhenso Victor Albites CondoriCopyright:
Available Formats
Repaglinide
O OH
N O
H
N
Chemical name: (S)-(+)-2-Ethoxy-4-[2-(3-methyl-1-[2-(piperidin-1-yl)
phenyl]butylamino)-2-oxoethyl]benzoic acid
CAS number: 135062-02-1
Molecular formula: C27 H36 N2 O4
Molecular mass (g mol−1 ): 452.6
InChI: 1S/C27H36N2O4/c1-4-33-25-17-20(12-13-22(25)27(31)32)
18-26(30)28-23(16-19(2)3)21-10-6-7-11-24(21)29-14-8-5-9-15-29/
h6-7,10-13,17,19,23H,4-5,8-9,14-16,18H2,1-3H3,(H,28,30)(H,31,32)/
t23-/m0/s1
InChIKey: FAEKWTJYAYMJKF-QHCPKHFHSA-N
Biological activity
Repaglinide (brand names: Prandin, GlucoNorm, NovoNorm, Sureposr, etc.) is a
short-acting antidiabetic drug. It lowers blood glucose by stimulating the release
of insulin from the pancreas. It is used in type II diabetic patients to normalize
postprandial hyperglycemia.
In vitro metabolism
The in vitro metabolism of [14 C]-repaglinide (1) was investigated in human liver
microsomes (HLMs) and recombinant cytochrome P450s (CYPs). Metabolites were
Handbook of Metabolic Pathways of Xenobiotics, First Edition.
Edited by Philip W. Lee, Hiroyasu Aizawa, Lawrence L. Gan, Chandra Prakash, and Dafang Zhong.
© 2014 John Wiley & Sons, Ltd. Published 2014 by John Wiley & Sons, Ltd.
2
O OH
OH O O
O O
Repaglinide
O OH O OH
O O OH
OH N O
N O N O
H H
H
N+ N
N 9 7 8
O O O
HO
O OH O OH O OH
N OH N O N O
H H H
N N N
5 1 6
O O O
O OH O OH O OH
N O N O N O
H H H
NH2 2 NH O N
OH
3 OH 4
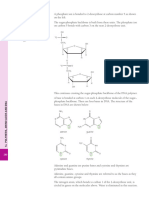
Scheme 1 In vitro metabolic pathway of repaglinide (1).
Repaglinide 3
measured by high-performance liquid chromatography (HPLC) with online radio-
chemical detection and identified by liquid chromatography–mass spectrometry
(LC-MS) and LC-MS-nuclear magnetic resonance (NMR). Five oxidative metabo-
lites, including an aromatic amine (2) and a dicarboxylic acid (3), both formed by
the opening of the piperidine ring; 4 yielded by hydroxylation of the piperidine
ring; 5 yielded by O-deethylation; and 6 yielded hydroxylation on the isopropyl
moiety. Metabolites 2 and 4 were the primary compounds found in HLMs. CYP2C8
catalyzed the formation of the major metabolite 4, and minor metabolites 2 and 6.
The major metabolite generated by CYP3A4 was 2, metabolites 3, 5, and 6 were
also detected.1
Metabolism of [3 H]-repaglinide in HLMs, human liver S9, human hepatocytes
and recombinant UDP-glucuronosyltransferases (UGTs), and CYPs were also stud-
ied. In addition to 2–6, two additional oxidative dehydrogenation metabolites (7
and 8) were found in HLMs. CYP3A4 mainly catalyzed the biotransformation of 1
to an intermediate (iminium or an aldehyde), which could be further oxidized by an
unidentified cytosolic enzyme to 3. Incubation of [3 H]-repaglinide (0.5 μM) with
O OH
O O O
OH
O O S
O N OH
H
O O OH
N O
OH H
N O
H N
N 9 10
O
O O
O OH
O OH O OH
N O
N OH N O H
H H
N
N N
5 1
4
OH
O
O
O OH
O OH
N O
N O H
H
NH O
NH2
OH
2 3
Scheme 2 In vivo metabolic pathway of repaglinide (1).
4 Repaglinide
human hepatocytes yielded 3 (11%), 4 (13%), and an acylglucuronide of repaglin-
ide 9 (12%) as the major metabolites. UGT1A1 catalyzed the formation of 9.2 The
in vitro metabolic pathway of repaglinide is shown in Scheme 1.
In vivo metabolism
After oral or intravenous (i.v.) administration of [14 C]-repaglinide (1), the metabolic
profiles in plasma, urine, and bile or feces of humans, cynomolgus monkeys,
dogs, rabbits, rats, and mice were analyzed using radio-HPLC and MS. Extensive
metabolism was observed. Metabolites 2, 3, 4, and 5, acylglucuronide conjugate
9, and tauride conjugate 10 were detected. None of these metabolites exhibited
glucose-lowering effect. Compounds 2 and 3 were the major metabolites detected
in humans, monkeys, and rabbits, whereas 3 and 9 were major metabolites in dogs
and rats.
Metabolism and excretion of [14 C]-repaglinide in healthy male volunteers were
investigated following multiple oral dosing of 2 mg (50 μCi).3 Metabolic profile
of 1 in plasma, urine, and feces was determined by HPLC with fraction collection
followed by liquid scintillation counting. The parent compound accounted for 61%
of the total radioactivity in plasma, followed by the major metabolite 3 (piperidine
ring opening, 11%) and minor metabolites 2, 4, 5, 9, and 10. Metabolite 3 was
the major metabolite in feces, with metabolites 2, 4, 5, 9, and 10 as minor prod-
ucts. Metabolites 2 (24%) and 3 (22%) were the major urinary metabolites with
metabolites 4, 5, 9, and 10 as minor products.3 The in vivo metabolic pathway of
repaglinide is shown in Scheme 2.
Data compiled by Juefang Ding
Shanghai Institute of Materia Medica, Chinese Academy of Sciences,
Shanghai, People’s Republic of China
References
1. T.B. Bidstrup, I. Bjornsdottir, U.G. Sidelmann, et al. CYP2C8 and CYP3A4 are the prin-
cipal enzymes involved in the human in vitro biotransformation of the insulin secretagogue
repaglinide. Br. J. Clin. Pharmacol. 2003, 56, 305.
2. J.P. Gan, W.Q. Chen, H. Shen, et al. Repaglinide–gemfibrozil drug interaction: inhibition
of repaglinide glucuronidation as a potential additional contributing mechanism. Br. J. Clin.
Pharmacol. 2010, 70, 870.
3. P.N. van Heiningen, V. Hatorp, K. Kramer Nielsen, et al. Absorption, metabolism and excre-
tion of a single oral dose of (14)C-repaglinide during repaglinide multiple dosing. Eur. J. Clin.
Pharmacol. 1999, 55, 521.
You might also like
- Presentazione Tesi Matteo CerrinaDocument82 pagesPresentazione Tesi Matteo CerrinaMatteo CerrinaNo ratings yet
- Exam 4 Sample ProblemsDocument2 pagesExam 4 Sample ProblemsTJ SmithNo ratings yet
- Formation of A Polynucleotide: A Condensation Reaction Takes PlaceDocument3 pagesFormation of A Polynucleotide: A Condensation Reaction Takes PlaceKate TaylorNo ratings yet
- Oxidation of Benzoin Into Benzil (N°39) : Tatiana Pachova BSC 2, Chemistry Assistant: Chandan Dey Sciences Ii - Lab. ADocument4 pagesOxidation of Benzoin Into Benzil (N°39) : Tatiana Pachova BSC 2, Chemistry Assistant: Chandan Dey Sciences Ii - Lab. ARabiaNo ratings yet
- Energy Production: Hapter 3Document18 pagesEnergy Production: Hapter 3Supriya A SNo ratings yet
- Organic Chemistry Mechanistic Patterns Canadian 1st Edition Ogilvie Solutions Manual 1Document32 pagesOrganic Chemistry Mechanistic Patterns Canadian 1st Edition Ogilvie Solutions Manual 1nancyyorkddsmcfjqakdow100% (26)
- Formation of Glucose 6-PhosphateDocument2 pagesFormation of Glucose 6-PhosphateSanjeefKumrIINo ratings yet
- 14glucose6 PpsDocument2 pages14glucose6 PpsSanjeefKumrIINo ratings yet
- Tetrodotoxin: TTX: BackgroundDocument12 pagesTetrodotoxin: TTX: BackgroundMarrauNo ratings yet
- 去Boc保护Document1 page去Boc保护angle6321No ratings yet
- Antibiotik Betha LaktamDocument18 pagesAntibiotik Betha LaktamdmujahidinNo ratings yet
- Organic Chemistry Mechanistic Patterns Canadian 1St Edition Ogilvie Solutions Manual Full Chapter PDFDocument36 pagesOrganic Chemistry Mechanistic Patterns Canadian 1St Edition Ogilvie Solutions Manual Full Chapter PDFmaria.topolosky417100% (11)
- DNA PPSXDocument19 pagesDNA PPSXTintin Brusola SalenNo ratings yet
- Zeocin ManDocument24 pagesZeocin ManThaís Paiva Porto de SouzaNo ratings yet
- Unit 3 QuestionsDocument18 pagesUnit 3 QuestionsLynd TaylorNo ratings yet
- APIChem Featured Products PDFDocument5 pagesAPIChem Featured Products PDFTezozómocNo ratings yet
- DNA ReplicationDocument20 pagesDNA ReplicationFrie An PanteNo ratings yet
- (Aqa A Level Science) Teresa Quigg - Aqa A Level Chemistry Studentbook 2 (Aqa A Level Science) - Hodder Education (2015)Document3 pages(Aqa A Level Science) Teresa Quigg - Aqa A Level Chemistry Studentbook 2 (Aqa A Level Science) - Hodder Education (2015)Dr kamruzzaman EyeNo ratings yet
- Lecture Notes Chem 51A S. King: O H OH H HO HO H H H CH OHDocument41 pagesLecture Notes Chem 51A S. King: O H OH H HO HO H H H CH OHTamiaNo ratings yet
- Legaturi glicozidice si structuri glucidice complexeDocument7 pagesLegaturi glicozidice si structuri glucidice complexedeedeeutza14No ratings yet
- Membuat Gambar Struktur Kimia dan Nama IUPAC Senyawa ObatDocument6 pagesMembuat Gambar Struktur Kimia dan Nama IUPAC Senyawa ObatRisky DaniarNo ratings yet
- Marine Chemical EcologyDocument33 pagesMarine Chemical EcologySofyan ZenNo ratings yet
- Chemistry NoteDocument27 pagesChemistry NoteUmar M abubakarNo ratings yet
- Grafic Sea Cucumber AntelminticDocument1 pageGrafic Sea Cucumber AntelminticFeraCygCatzNo ratings yet
- Mcmurry PDFDocument119 pagesMcmurry PDFRicardo SierraNo ratings yet
- 2006-CHM6108 - L5L6 HandoutDocument9 pages2006-CHM6108 - L5L6 Handoutaidar.seralinNo ratings yet
- Practical FCHG412 Prodrugs: Module Outcomes For The PracticalDocument14 pagesPractical FCHG412 Prodrugs: Module Outcomes For The PracticalGreg RalphNo ratings yet
- E12 AtqDocument5 pagesE12 AtqCharlene InaoNo ratings yet
- Synthesis of amino carbonyl compounds via reductive aminationDocument6 pagesSynthesis of amino carbonyl compounds via reductive aminationNguyễn Đức DuyNo ratings yet
- Molecules: Design, Synthesis, Antinociceptive and Anti-Inflammatory Activities of Novel Piroxicam AnaloguesDocument20 pagesMolecules: Design, Synthesis, Antinociceptive and Anti-Inflammatory Activities of Novel Piroxicam AnaloguesPriyosetyokoNo ratings yet
- Substrate Synthesis Routes ComparisonDocument2 pagesSubstrate Synthesis Routes ComparisonsauronsauronNo ratings yet
- Conjugated Bile Salts Liaflet 2020Document2 pagesConjugated Bile Salts Liaflet 2020Balachandar BNo ratings yet
- Medicinal Chemistry & Drug Discovery: Dr. Peter Wipf Department of Chemistry University of PittsburghDocument69 pagesMedicinal Chemistry & Drug Discovery: Dr. Peter Wipf Department of Chemistry University of PittsburghKhushi KhanNo ratings yet
- Ecteinascidin 743 (080414-TKGP) synthesis highlightsDocument3 pagesEcteinascidin 743 (080414-TKGP) synthesis highlightsPercival GalahadNo ratings yet
- Alcohols-Structure and Synthesis 2Document82 pagesAlcohols-Structure and Synthesis 2Ali Issa OthmanNo ratings yet
- Chemistry SynopsisDocument7 pagesChemistry SynopsisDr Prashant Shihora100% (1)
- 1 s2.0 S0960894X09013183 mmc1Document7 pages1 s2.0 S0960894X09013183 mmc1Gourab SahaNo ratings yet
- K7 - Metabolisme NukleotidaDocument33 pagesK7 - Metabolisme NukleotidaAditya MuchayatsyahNo ratings yet
- K7 - Metabolisme NukleotidaDocument32 pagesK7 - Metabolisme NukleotidaTiara YosephaNo ratings yet
- Structure, Transcription and EditingDocument12 pagesStructure, Transcription and EditingTintin Brusola SalenNo ratings yet
- The Chemistry of PoppiesDocument1 pageThe Chemistry of PoppiesShruti SinghalNo ratings yet
- Carbohydrates: Answers To QuestionsDocument3 pagesCarbohydrates: Answers To QuestionsGaby de GuzmanNo ratings yet
- Research ArticleDocument7 pagesResearch Articleana ulfaNo ratings yet
- Chemical Constituents of The Mexican Mistletoe (Psittacanthus Calyculatus)Document7 pagesChemical Constituents of The Mexican Mistletoe (Psittacanthus Calyculatus)Educación UniversidadNo ratings yet
- Antibiotics Actions, Origins, Resistance by Christopher Walsh (Z-Lib - Org) - 79-86Document8 pagesAntibiotics Actions, Origins, Resistance by Christopher Walsh (Z-Lib - Org) - 79-86British PropolisNo ratings yet
- Alkaloid S1Document142 pagesAlkaloid S1Faisal MNo ratings yet
- Enfermagem AvançadaDocument48 pagesEnfermagem AvançadaenfamandaramalhoNo ratings yet
- Osteoprorosis Therapy Management, Beyond Safety and Efficacy (Once Yearly Treatment)Document29 pagesOsteoprorosis Therapy Management, Beyond Safety and Efficacy (Once Yearly Treatment)ian ismeNo ratings yet
- Macrolide Antibiotics: Binding Site, Mechanism of Action, ResistanceDocument15 pagesMacrolide Antibiotics: Binding Site, Mechanism of Action, ResistancevitulNo ratings yet
- What Is Organic Chemistry?Document72 pagesWhat Is Organic Chemistry?gs7514424No ratings yet
- Classes of Antibiotics PDFDocument1 pageClasses of Antibiotics PDFVương TúNo ratings yet
- 5.36 Biochemistry Laboratory: Mit OpencoursewareDocument11 pages5.36 Biochemistry Laboratory: Mit OpencoursewareNeenu RajputNo ratings yet
- Nucleic Acid Structure & DNA ReplicationDocument51 pagesNucleic Acid Structure & DNA ReplicationJMCDUFFIENo ratings yet
- Progress Report of Anamica T3Document2 pagesProgress Report of Anamica T3Jonathan HicksNo ratings yet
- (S) - 5 - ( (S) - 1,2-Dihydroxyethyl) - 3,4-Dihydroxyfuran-2 (5H) - One: OH HODocument2 pages(S) - 5 - ( (S) - 1,2-Dihydroxyethyl) - 3,4-Dihydroxyfuran-2 (5H) - One: OH HOJoz MtzNo ratings yet
- Proline PDFDocument52 pagesProline PDFRathinNo ratings yet
- Substrate-B reference compoundDocument2 pagesSubstrate-B reference compoundsauronsauronNo ratings yet
- RNA Structure, Components, Transcription and Editing ProcessDocument12 pagesRNA Structure, Components, Transcription and Editing ProcessChristian Jim PollerosNo ratings yet
- Artículo 2-OhDocument10 pagesArtículo 2-OhRhenso Victor Albites CondoriNo ratings yet
- Nanotechnology & DermatologyDocument11 pagesNanotechnology & Dermatologyshiva100% (1)
- Code RCH 2003Document23 pagesCode RCH 2003Rhenso Victor Albites CondoriNo ratings yet
- Jcad 12 7 34Document17 pagesJcad 12 7 34Rizki MohamadNo ratings yet
- Fluconazole But Not Itraconazole Decreases The Metabolism of Losartan To E-3174Document5 pagesFluconazole But Not Itraconazole Decreases The Metabolism of Losartan To E-3174Rhenso Victor Albites CondoriNo ratings yet
- HLB & Emulsion StabilityDocument18 pagesHLB & Emulsion Stabilityvidya JainNo ratings yet
- 10.1208@s12248 019 0382 2Document12 pages10.1208@s12248 019 0382 2Rhenso Victor Albites CondoriNo ratings yet
- The Influence of Surfactant HLB and Oil-SurfactantDocument12 pagesThe Influence of Surfactant HLB and Oil-SurfactantRhenso Victor Albites CondoriNo ratings yet
- HLB & Emulsion StabilityDocument18 pagesHLB & Emulsion Stabilityvidya JainNo ratings yet
- Role of miRNAs in treatment response and toxicity of childhood acute lymphoblastic leukemiaDocument14 pagesRole of miRNAs in treatment response and toxicity of childhood acute lymphoblastic leukemiaRhenso Victor Albites CondoriNo ratings yet
- NII-Electronic Library ServiceDocument5 pagesNII-Electronic Library ServiceRhenso Victor Albites CondoriNo ratings yet
- Lycopene and Melatonin: Antioxidant Compounds in Cosmetic FormulationsDocument7 pagesLycopene and Melatonin: Antioxidant Compounds in Cosmetic FormulationsRhenso Victor Albites Condori100% (1)
- Identity of Antibiotic P-42-1 Elaborated by Actinomyces tumemaceransDocument5 pagesIdentity of Antibiotic P-42-1 Elaborated by Actinomyces tumemaceransRhenso Victor Albites CondoriNo ratings yet
- Cheng, M. M., & Humphreys, K. A.Document25 pagesCheng, M. M., & Humphreys, K. A.Rhenso Victor Albites CondoriNo ratings yet
- BAM Chapter 23 - Methods For Cosmetics - FDADocument11 pagesBAM Chapter 23 - Methods For Cosmetics - FDARhenso Victor Albites CondoriNo ratings yet
- Toxicidad de PasifloraDocument5 pagesToxicidad de PasifloraRhenso Victor Albites CondoriNo ratings yet
- Jordi Perramon - AministracionDocument20 pagesJordi Perramon - AministracionRhenso Victor Albites CondoriNo ratings yet
- Omeike2019 Article PotentialAntibiotic-producingFDocument7 pagesOmeike2019 Article PotentialAntibiotic-producingFRhenso Victor Albites CondoriNo ratings yet
- Repaglinide: Chemical Name: (S) - (Document4 pagesRepaglinide: Chemical Name: (S) - (Rhenso Victor Albites CondoriNo ratings yet
- Hipoglucemia de MaracuyaDocument7 pagesHipoglucemia de MaracuyaRhenso Victor Albites CondoriNo ratings yet
- Huella Digital de PasifloraDocument9 pagesHuella Digital de PasifloraRhenso Victor Albites CondoriNo ratings yet
- In Vitro Studies On Physiological and Chemical Stability of New LE404-Derivatives With Extended Half-LifeDocument7 pagesIn Vitro Studies On Physiological and Chemical Stability of New LE404-Derivatives With Extended Half-LifeRhenso Victor Albites CondoriNo ratings yet
- Hongo Endofítico Productor de Antimicrobianos VolátilesDocument10 pagesHongo Endofítico Productor de Antimicrobianos VolátilesRhenso Victor Albites CondoriNo ratings yet
- Exopolysaccharide of Nostoc muscorum increases soil aggregationDocument5 pagesExopolysaccharide of Nostoc muscorum increases soil aggregationRhenso Victor Albites CondoriNo ratings yet
- Expanding the Ubiquitin Code Through Post-Translational ModificationDocument13 pagesExpanding the Ubiquitin Code Through Post-Translational ModificationRhenso Victor Albites CondoriNo ratings yet
- Problemas de TabletasDocument59 pagesProblemas de TabletasLucho Hualan SandovalNo ratings yet
- TRB 4 PabliDocument10 pagesTRB 4 PabliRhenso Victor Albites CondoriNo ratings yet
- Advances in Biochemical Engineering Biotechnology 066 - Bioanalysis and Biosensors For Bioprocess MonitoringDocument236 pagesAdvances in Biochemical Engineering Biotechnology 066 - Bioanalysis and Biosensors For Bioprocess MonitoringGhaier KazmiNo ratings yet
- 539875Document254 pages539875Johnny Atman100% (1)
- PHOTOSYNTHESIS-Student Worksheet (With Answers)Document8 pagesPHOTOSYNTHESIS-Student Worksheet (With Answers)Daniella Gabrielle Gerodias50% (10)
- Maricris Q. Marquita-Uy R.N, M.DDocument42 pagesMaricris Q. Marquita-Uy R.N, M.DAdrian Mai AlanNo ratings yet
- A Mi No AcidsDocument61 pagesA Mi No AcidsWilder VargasNo ratings yet
- PharmD 1st Year SyllabusDocument21 pagesPharmD 1st Year SyllabusAshwat ANo ratings yet
- Lipid Metabolism PDFDocument40 pagesLipid Metabolism PDFQashas OktaniaNo ratings yet
- Medical Biochemistry Course OverviewDocument9 pagesMedical Biochemistry Course OverviewFaridaNo ratings yet
- Boce3714 Weekly Assessment 1Document2 pagesBoce3714 Weekly Assessment 1ayanda thelaNo ratings yet
- Human Physiology & Bio-ChemistryDocument22 pagesHuman Physiology & Bio-ChemistryPranav PawarNo ratings yet
- Wort Composition and Its Impact On The Flavour-Active Higher Alcohol and Ester Formation of Beer - A ReviewDocument7 pagesWort Composition and Its Impact On The Flavour-Active Higher Alcohol and Ester Formation of Beer - A ReviewGSBYGALATINo ratings yet
- ATP Photosynthesis Respiration Webquest ReviewDocument2 pagesATP Photosynthesis Respiration Webquest ReviewAlayna SheltonNo ratings yet
- Photosynthesis PDFDocument22 pagesPhotosynthesis PDFbhaskar rayNo ratings yet
- Lesson 1-2Document7 pagesLesson 1-2Sophia DGNo ratings yet
- FullDocument2,821 pagesFullSemuel RiakNo ratings yet
- Photosynthesis Diagrams WorksheetDocument2 pagesPhotosynthesis Diagrams WorksheetG Meera Prem Kumar20% (5)
- Anaerobic Tennis Ball LabDocument5 pagesAnaerobic Tennis Ball Labapi-30432692050% (2)
- BABS1201-Study-Notes UNSWdocDocument28 pagesBABS1201-Study-Notes UNSWdocgiraffequeenNo ratings yet
- ATP Generation in MetabolismDocument2 pagesATP Generation in MetabolismChristopher GalivoNo ratings yet
- 2.7 (BIOCHEMISTRY) Gluconeogenesis - Better PicturesDocument12 pages2.7 (BIOCHEMISTRY) Gluconeogenesis - Better Pictureslovelots1234No ratings yet
- Essential Elements and Their Distribution in The ProfileDocument25 pagesEssential Elements and Their Distribution in The ProfileFeodore Abrion ReplikoNo ratings yet
- 5850 5858 Absorption Metabolism and Protective Role of Fruits and Vegetables Polyphenols Against Gastric CancerDocument9 pages5850 5858 Absorption Metabolism and Protective Role of Fruits and Vegetables Polyphenols Against Gastric CancerMonaNo ratings yet
- Nitrogen CycleDocument6 pagesNitrogen CycleAngelica Soriano100% (1)
- James - Krieger - The - Metabolism - MythDocument68 pagesJames - Krieger - The - Metabolism - MythArthur G T DiehlNo ratings yet
- Healthy Habits KitDocument2 pagesHealthy Habits KitAuraLoreNo ratings yet
- Part I - Too-Weighty-OneDocument5 pagesPart I - Too-Weighty-OneRosemarie R. Reyes100% (2)
- Dintzis ExerciseDocument5 pagesDintzis ExerciserichschurNo ratings yet
- Biosci 3Document15 pagesBiosci 3MARJORYNo ratings yet
- MCQs in BiochemistryDocument322 pagesMCQs in Biochemistryjayrajsen100% (2)
- Chemvera Speciality - Food Ingredient & NutraceuticalDocument61 pagesChemvera Speciality - Food Ingredient & NutraceuticalsambhavjoshiNo ratings yet
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Coating and Drying Defects: Troubleshooting Operating ProblemsFrom EverandCoating and Drying Defects: Troubleshooting Operating ProblemsRating: 5 out of 5 stars5/5 (1)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireFrom EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireRating: 4 out of 5 stars4/5 (129)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Gas-Liquid And Liquid-Liquid SeparatorsFrom EverandGas-Liquid And Liquid-Liquid SeparatorsRating: 3.5 out of 5 stars3.5/5 (3)
- Chemistry: 1001 Practice Problems For Dummies (+ Free Online Practice)From EverandChemistry: 1001 Practice Problems For Dummies (+ Free Online Practice)No ratings yet
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Transformer: The Deep Chemistry of Life and DeathFrom EverandTransformer: The Deep Chemistry of Life and DeathRating: 4.5 out of 5 stars4.5/5 (13)