Professional Documents
Culture Documents
Lecture31 Wed Nov 29
Uploaded by
Akib ImtihanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lecture31 Wed Nov 29
Uploaded by
Akib ImtihanCopyright:
Available Formats
5.
13
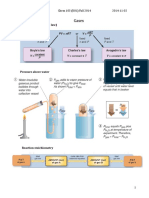
5C.1(c) The Lever Rule
How do we determine the amount in each phase?
Consider benzene:
total g l
nbenzene = nbenzene + nbenzene
However, we can relate the number of moles of benzene in each phase to the total number of moles and
the corresponding mole fractions, i.e.,
n total zbenzene = n g ybenzene + n l xbenzene
total l g
Since, n = n + n , we can replace the number of moles in the liquid to write:
n total zbenzene = n g ybenzene + (n total − n g)xbenzene
or
n total(zbenzene − xbenzene) = n g(ybenzene − xbenzene) [1]
Similarly, we can replace the number of moles in the gas phase to write:
n total zbenzene = (n total − n l )ybenzene + n l xbenzene
or
n total(zbenzene − ybenzene) = n l(xbenzene − ybenzene) [2]
Taking the ratio of [1] and [2], we obtain:
(zbenzene − xbenzene) n g(ybenzene − xbenzene)
=
(zbenzene − ybenzene) n l(xbenzene − ybenzene)
or, rearranging
ng (zbenzene − xbenzene)
= [Lever Rule]
nl (ybenzene − zbenzene)
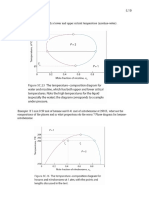
5C.2 Temperature-composition diagrams
Since the vapour pressure of each component changes with temperature, we must also look at the
behaviour of the boiling temperature as the composition of the liquid solution varies at fixed
pressure. Therefore, as we'll see, it is often much more useful to look at the T/composition plot
[NOTE: liquid phase is now on the bottom and vapour on the top]
5.14
5C.2(a) Distillation
Consider the diagram above and the following process to separate the two liquids:
(i) Mixture of composition a1 is heated until it reaches it boiling point T2.
(ii) St the boiling point the liquid has composition a 2 = a1 while the vapour has composition
a′2 > a 2 (it is enriched in the more volatile liquid).
(iii) Collect the vapour and then cool to form liquid. Reheat to boiling point and then liquid has
composition a3 = a′2 while the vapour has composition a′3 > a3
(iv) Cycle as many times as needed to obtain desired purity of A.
5C.2(b) Azeotropes
We have seen that positive and negative deviations from Raoult’s law are possible in real
solutions. These deviations have a profound effect on vapour pressure and liquid vapour
equilibria.
(a) Negative deviation from Raoult’s Law, where there is significant interaction (H-bonding)
between the components, e.g., acetone-chloroform
• In the low A content region, gas is leaner in A than the liquid
• In the high A content region, gas is richer in A than the liquid
• The two regions meet at a point called the azeotrope where the gas and the liquid
have the same compositions; call the azeotrope: xchloroform = ychloroform = 0.35
5.15
(b) Positive deviation from Raoult’s Law, e.g., 1-propanol-water
• In the low A content region, gas is richer in A than the liquid - at low propanol mol
fraction, vapor is rich in propanol.
• In the high A content region, gas is less rich in A than the liquid - At high propanol mol
fraction, vapor is poor in propanol.
• The two regions meet at a point called the azeotrope where the gas and the liquid have
the same compositions. xpropanol = ypropanol = 0.432
You might also like
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsFrom EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsRating: 5 out of 5 stars5/5 (1)
- Recommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsFrom EverandRecommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsE. F. G. HeringtonNo ratings yet
- Chemistry General Sem IV Part 1Document10 pagesChemistry General Sem IV Part 1Cristiano RonaldoNo ratings yet
- Solution Solvent 0 SolventDocument7 pagesSolution Solvent 0 SolventTRÂN NGUYỄN NGỌC BẢONo ratings yet
- Chp10 Notes-PHASE EQUI PrintDocument24 pagesChp10 Notes-PHASE EQUI PrintNurul FarhanaNo ratings yet
- The Boiling Pointcomposition Diagram of Immiscible and Partially Miscible Liquid Systems1Document2 pagesThe Boiling Pointcomposition Diagram of Immiscible and Partially Miscible Liquid Systems1Ronald Cedric RadaNo ratings yet
- Multicomponent DistillationDocument10 pagesMulticomponent DistillationDAMP ChemicalNo ratings yet
- Properties of Pure SubstancesDocument11 pagesProperties of Pure SubstancesTirth SolankiNo ratings yet
- Chapter 1 DistillationDocument110 pagesChapter 1 DistillationSiti Nurshahira80% (5)
- Chapter 8 Phase DiagramsDocument18 pagesChapter 8 Phase DiagramsWynlor AbarcaNo ratings yet
- Chapter - 2 DistillationDocument75 pagesChapter - 2 DistillationJACOB DAVENo ratings yet
- CMT 405 - Distillation PDFDocument72 pagesCMT 405 - Distillation PDFMuhammad Azri HaziqNo ratings yet
- More Colligative Properties, Solutions & Distillation: Class 4.2Document7 pagesMore Colligative Properties, Solutions & Distillation: Class 4.2Roberto CastilhoNo ratings yet
- Lecture 1Document31 pagesLecture 1Nimit RiniNo ratings yet
- Chapter 1 - Distillation PDFDocument107 pagesChapter 1 - Distillation PDFFatin Natasha NazriNo ratings yet
- Key Concepts: TotalDocument18 pagesKey Concepts: TotalSachin Kumar67% (3)
- Design Techniques To AbsorptionDocument54 pagesDesign Techniques To AbsorptionFASIH AFZAL KHANNo ratings yet
- Destilation: EVEN SEMESTER 2013/2014Document24 pagesDestilation: EVEN SEMESTER 2013/2014انس خيرناNo ratings yet
- Semi-batch reactor conversionDocument5 pagesSemi-batch reactor conversionDheeraj ShuklaNo ratings yet
- Transcript - Multicomponent Flash Calculations VideoDocument3 pagesTranscript - Multicomponent Flash Calculations VideoChristopher RileyNo ratings yet
- Che 312 Notebook DistillationDocument19 pagesChe 312 Notebook DistillationUGWU MARYANNNo ratings yet
- Multiphase SystemDocument93 pagesMultiphase SystemNoorhalieza AliNo ratings yet
- 3 Thermodynamic Properties of Pure SubstancesDocument14 pages3 Thermodynamic Properties of Pure SubstancesLexNo ratings yet
- Phase Equilibria: One-Component Systems Solutions of NonelectrolytesDocument50 pagesPhase Equilibria: One-Component Systems Solutions of NonelectrolyteskarinaNo ratings yet
- Colligative Properties: Nathaniel P. DugosDocument32 pagesColligative Properties: Nathaniel P. DugossololexzibNo ratings yet
- Solutions FinalDocument12 pagesSolutions FinalAman DeepNo ratings yet
- Lecture #2-Physical Chemistry - 8 (L2-P-2)Document10 pagesLecture #2-Physical Chemistry - 8 (L2-P-2)احمد الدلالNo ratings yet
- Contact Equilibrium ProcessesDocument13 pagesContact Equilibrium ProcessesDavid Aremania100% (1)
- Calculate heat duty for mixing H2SO4 and H2ODocument4 pagesCalculate heat duty for mixing H2SO4 and H2OAkash RaoNo ratings yet
- Heat of MxingDocument4 pagesHeat of MxingAkash RaoNo ratings yet
- Physical Chemistry II (TKK-1237) : Review ReviewDocument7 pagesPhysical Chemistry II (TKK-1237) : Review ReviewUlvatus Sa' DiyahNo ratings yet
- DocxDocument11 pagesDocxNabila PutriNo ratings yet
- Colligative Properties and Determination of Molar MassesDocument7 pagesColligative Properties and Determination of Molar MassesRoshini FelixNo ratings yet
- ENVE_212_week 6,7_spring2023_24_1Document41 pagesENVE_212_week 6,7_spring2023_24_1yasemin yılmazNo ratings yet
- Colligative Properties 2Document10 pagesColligative Properties 2lilik supriantiNo ratings yet
- Che 411-Vapour Pressure and BoilingDocument17 pagesChe 411-Vapour Pressure and BoilingHannah CokerNo ratings yet
- Chapter 3Document61 pagesChapter 3rejie magnayeNo ratings yet
- Phase Diagrams of Mixtures ExplainedDocument24 pagesPhase Diagrams of Mixtures ExplainedmohammedNo ratings yet
- CPE1002 Chap13 2011Document37 pagesCPE1002 Chap13 2011Shazriel Yushamiel YusoffNo ratings yet
- 4-CHAPTER 3a-PVTDocument37 pages4-CHAPTER 3a-PVTMahamed HusseinNo ratings yet
- Chapter ThreeDocument33 pagesChapter ThreeDheyaa Al-JubouriNo ratings yet
- Claysius Clapeyron Lab ExperimentDocument11 pagesClaysius Clapeyron Lab Experimentmohamad munzir100% (1)
- Liquid Solution (13th)Document19 pagesLiquid Solution (13th)Raju SinghNo ratings yet
- CHEM 254 Experiment 8 Phase Diagrams - Liquid Vapour Equilibrium For Two Component SolutionsDocument6 pagesCHEM 254 Experiment 8 Phase Diagrams - Liquid Vapour Equilibrium For Two Component SolutionsAsilahNo ratings yet
- Lecture 3 - Systems (SFEE) Specific Heats The Working FluidDocument38 pagesLecture 3 - Systems (SFEE) Specific Heats The Working FluidWillie MojataleNo ratings yet
- Distillation IDocument57 pagesDistillation IOsan ThorpeNo ratings yet
- Report1 Draft2 27-9Document36 pagesReport1 Draft2 27-9Arvind RavichandranNo ratings yet
- Phase EquilibriaDocument21 pagesPhase EquilibriasuperchellyNo ratings yet
- Wa0245 1Document45 pagesWa0245 1lm7032478No ratings yet
- Phase DiagramsDocument19 pagesPhase Diagramsget2csNo ratings yet
- Distillation Lecture Note-2Document20 pagesDistillation Lecture Note-2BasseyNo ratings yet
- Pemisahan Kimia: Materi 4: DistilasiDocument50 pagesPemisahan Kimia: Materi 4: DistilasiMochammad Syehfu Aref GhozaliNo ratings yet
- DistillationDocument103 pagesDistillationKrishnamoorthy VijayalakshmiNo ratings yet
- 3 - Properties of Pure SubstancesDocument34 pages3 - Properties of Pure SubstancesRoman MiaNo ratings yet
- Binary distillation designDocument23 pagesBinary distillation designPar PatelNo ratings yet
- Gas Absorption and Gas StrippingDocument14 pagesGas Absorption and Gas StrippingEK63No ratings yet
- Colligative PropertiesDocument7 pagesColligative Propertiesakshatjn100% (1)
- Properties of Pure SubstanceDocument9 pagesProperties of Pure Substanceashenafihenok400No ratings yet
- Chemistry UNIT 4 NotesDocument10 pagesChemistry UNIT 4 NotesSammit NadkarniNo ratings yet
- CHE 374 Computational Methods Class Examples 2.2Document1 pageCHE 374 Computational Methods Class Examples 2.2Akib ImtihanNo ratings yet
- Chem 10X Data Sheet: 1. Periodic Table of The ElementsDocument2 pagesChem 10X Data Sheet: 1. Periodic Table of The ElementsAkib ImtihanNo ratings yet
- Sample First Midterm Exam SolutionsDocument5 pagesSample First Midterm Exam SolutionsAkib ImtihanNo ratings yet
- Gases: Ideal Gases (Ideal Gas Law)Document2 pagesGases: Ideal Gases (Ideal Gas Law)Akib ImtihanNo ratings yet
- Determining dominant intermolecular forcesDocument1 pageDetermining dominant intermolecular forcesAkib ImtihanNo ratings yet
- CHE 374 Computational Methods Class Examples 2.2Document1 pageCHE 374 Computational Methods Class Examples 2.2Akib ImtihanNo ratings yet
- CHE 374 Computational Methods in Engineering: NumbersDocument6 pagesCHE 374 Computational Methods in Engineering: NumbersAkib ImtihanNo ratings yet
- MATLAB Basics: CHE 374 Computational Methods in EngineeringDocument10 pagesMATLAB Basics: CHE 374 Computational Methods in EngineeringAkib ImtihanNo ratings yet
- Sample First Midterm Exam CHEM 101/103, Section Z9 Date of Exam, 12:00 - 12:45Document6 pagesSample First Midterm Exam CHEM 101/103, Section Z9 Date of Exam, 12:00 - 12:45Akib ImtihanNo ratings yet
- CHE 374 - Computational Methods in Engineering: Topic Chapter Ref. in TextbookDocument1 pageCHE 374 - Computational Methods in Engineering: Topic Chapter Ref. in TextbookAkib ImtihanNo ratings yet
- Rules For Significant Figures/DigitsDocument5 pagesRules For Significant Figures/DigitsAkib ImtihanNo ratings yet
- DEs IVP2018 4upDocument4 pagesDEs IVP2018 4upAkib ImtihanNo ratings yet
- MATLAB - OnTheHub - License-Download-Install GuideDocument2 pagesMATLAB - OnTheHub - License-Download-Install GuideAkib ImtihanNo ratings yet
- Computational Methods in Engineering Che 374Document6 pagesComputational Methods in Engineering Che 374Akib ImtihanNo ratings yet
- CH E 374 Computational Methods in Engineering Course OutlineDocument4 pagesCH E 374 Computational Methods in Engineering Course OutlineAkib ImtihanNo ratings yet
- Section 2D. State Functions and Exact DifferentialsDocument4 pagesSection 2D. State Functions and Exact DifferentialsAkib ImtihanNo ratings yet
- Lecture35 Fri Dec 8Document3 pagesLecture35 Fri Dec 8Akib ImtihanNo ratings yet
- Taylor SeriesDocument2 pagesTaylor SeriesAkib ImtihanNo ratings yet
- Section 3: The Second and Third Laws Section 3A EntropyDocument2 pagesSection 3: The Second and Third Laws Section 3A EntropyAkib ImtihanNo ratings yet
- Calculating Temperature Change for Adiabatic Expansion of GasDocument3 pagesCalculating Temperature Change for Adiabatic Expansion of GasAkib ImtihanNo ratings yet
- P NRT V Bar DM K MolDocument2 pagesP NRT V Bar DM K MolAkib ImtihanNo ratings yet
- Distillation: A Aazeo A AazeoDocument3 pagesDistillation: A Aazeo A AazeoAkib ImtihanNo ratings yet
- Lecture34 Wed Dec 6Document3 pagesLecture34 Wed Dec 6Akib ImtihanNo ratings yet
- Lecture33 Mon Dec 4Document3 pagesLecture33 Mon Dec 4Akib ImtihanNo ratings yet
- Maxwell-Boltzmann, real gas behavior, virial coefficientsDocument4 pagesMaxwell-Boltzmann, real gas behavior, virial coefficientsAkib ImtihanNo ratings yet
- P F A NMV V P NM V V V: Example: Calculate The Rms Speed of He Atoms at 298 KDocument3 pagesP F A NMV V P NM V V V: Example: Calculate The Rms Speed of He Atoms at 298 KAkib ImtihanNo ratings yet
- H H C O mol C O mol: H H + Δ H + Δ H = 7.5192 kJ + 40.656 kJ − 3.358 kJ = 44.83 kJDocument5 pagesH H C O mol C O mol: H H + Δ H + Δ H = 7.5192 kJ + 40.656 kJ − 3.358 kJ = 44.83 kJAkib ImtihanNo ratings yet
- Lecture7 Wed Sep 20Document5 pagesLecture7 Wed Sep 20Akib ImtihanNo ratings yet
- Van der Waals gas equation of stateDocument2 pagesVan der Waals gas equation of stateAkib ImtihanNo ratings yet
- Fluorescence Spectroscopy: CHE5540 Lab Exercise 9Document10 pagesFluorescence Spectroscopy: CHE5540 Lab Exercise 9prakush_prakushNo ratings yet
- Module 2: Sulfur and Its Compounds: Burning of Raw Sulfur Roasting of Pyrites Production of Sulfuric AcidDocument6 pagesModule 2: Sulfur and Its Compounds: Burning of Raw Sulfur Roasting of Pyrites Production of Sulfuric AcidKhristel PenoliarNo ratings yet
- Science Revision Sheet - Term 1-Year 4: NameDocument18 pagesScience Revision Sheet - Term 1-Year 4: NameSaid HusseinNo ratings yet
- Sample ReportDocument39 pagesSample Reportgerman esteban rodriguez baqueroNo ratings yet
- AISI 410 Stainless SteelDocument2 pagesAISI 410 Stainless SteelSreenubabu KandruNo ratings yet
- Product Bulletin: (Microcrystalline Zinc-PhosphateDocument2 pagesProduct Bulletin: (Microcrystalline Zinc-PhosphateJason SonidoNo ratings yet
- Nucleus and RibosomesDocument2 pagesNucleus and RibosomesMark Lorenz LorillaNo ratings yet
- Food Tests Lab ReportDocument4 pagesFood Tests Lab ReportHirko BelayNo ratings yet
- Onuekwusi Et Al., 2014-1Document10 pagesOnuekwusi Et Al., 2014-1Cho ChoNo ratings yet
- Biology Yearly Lesson Plan Form 4Document4 pagesBiology Yearly Lesson Plan Form 4Hisyam Deraman100% (1)
- GenBio 1 - 2nd Quarter ReviewerDocument8 pagesGenBio 1 - 2nd Quarter Reviewerjoshua tejadaNo ratings yet
- Physical States & Types of Food DispersionsDocument13 pagesPhysical States & Types of Food DispersionsKhaled Abu-AlruzNo ratings yet
- h1536731881f9789386339430 - Old PDFDocument189 pagesh1536731881f9789386339430 - Old PDFAver BatthNo ratings yet
- RSE-SEE5 Circular1 2Document3 pagesRSE-SEE5 Circular1 2dD 16No ratings yet
- Introduction To Schiff BasesDocument2 pagesIntroduction To Schiff Basespulimamidi SarithareddyNo ratings yet
- ChiralVision Product ListDocument23 pagesChiralVision Product Listnilesh_rukeNo ratings yet
- (IIT Kharagpur Research Monograph Series 6) Mitra, Rahul - Structural Intermetallics and Intermetallic Matrix Composites-CRC Press (2015)Document324 pages(IIT Kharagpur Research Monograph Series 6) Mitra, Rahul - Structural Intermetallics and Intermetallic Matrix Composites-CRC Press (2015)Joshua Amalraj100% (1)
- Water Problems SolutionsDocument6 pagesWater Problems SolutionsHarshitha LokeshNo ratings yet
- Tectyl 502 CDocument2 pagesTectyl 502 CKARTHIGEYAN.RNo ratings yet
- Chem - Paper-I Ifs 2018Document6 pagesChem - Paper-I Ifs 2018ashishNo ratings yet
- Fandaruff (2014)Document8 pagesFandaruff (2014)Paulo DantasNo ratings yet
- Shell Tellus S2M68 Msds - 00107669Document7 pagesShell Tellus S2M68 Msds - 00107669Jasmine TsoNo ratings yet
- Acs JPCC 1c02131Document9 pagesAcs JPCC 1c02131arkaphysicsNo ratings yet
- CBM 2016 1Document14 pagesCBM 2016 1SHEIKH MUHAMMAD FAHADNo ratings yet
- Anuj AgrawalDocument15 pagesAnuj AgrawalSAVITRI GURJARNo ratings yet
- Nessler Reagent PDFDocument14 pagesNessler Reagent PDFBernardoNo ratings yet
- Simulaciones Numericas Detalladas de Reactores de Lechofijo CataliticoDocument13 pagesSimulaciones Numericas Detalladas de Reactores de Lechofijo CataliticoSanchez JorgeNo ratings yet
- Astm C1097Document1 pageAstm C1097pfta_No ratings yet
- CMC Guidelines On Hazardous Chemicals SpillsDocument8 pagesCMC Guidelines On Hazardous Chemicals Spillskhairul danialNo ratings yet
- Discovery of IonsDocument10 pagesDiscovery of Ionsekadarma55No ratings yet