Professional Documents
Culture Documents
Scan 26-Aug-2020 PDF
Uploaded by
chhaayaachitran akshuOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Scan 26-Aug-2020 PDF
Uploaded by
chhaayaachitran akshuCopyright:
Available Formats
196
UNDERGRADUATE ORGANIC CHEMISTAy
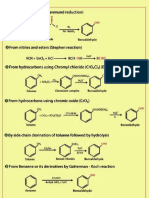
Condensation of urea with substituted
malonic esters
compounds called barbiturates. produces a clae.
Barbiturates
character. They from salts with acids. Free
are
cyclic diimides and are acidie
barbiturates and their salts
sedatives, soporifics and anesthetics. are
The familiar drugs barbitones,
barbiturates and may be
phenobarbital and
pentobarbital (Nembutal)
synthesised as below are
i) C2H5O
CHs COOEt
CzHOH CHs C-NH
coOEN-C--NH,-
CH (i) H2O
C2H NH 0
Barbitone
i) CaHO/
CgHs COOEt
NH2 C=0
NH2 C2H5OH CHs C-NH
C2Hs COOEt NH2 i) H2o H -NH
C=0
Diethyl ethyl phenyl
malonate O
Phenobarbital
CH3
(CH2)2
CH3-CHH COOEt
CaHs cOOEt NH2c0
NH2
Diethyl ethyl (1-methylbutyl)
malonate
6) CzH,oCH,OH
i) H2O
CH3
(CHa)
CH-CH. C-NH
NHCo
CaHs C-NH
O
Nembutal
5.4 DITHIANES
The sulphur atom of thiol is far better nucleophile than the oxygen atom of an
alcohol. Thiols add to the carbonyl group of aldehydes and ketones to form
tetrahedral carbonyl addition compounds. A common and most useful sulphur
nucleophile used for this purpose is 1, 3-propanedithiol. The carbonyl group of both
aldehydes and ketones react with this compound in the presence of an acid catalyst
to form cyclic thio acetals which is called 1, 3-dithianes.
You might also like
- Compound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesFrom EverandCompound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesNo ratings yet
- Scan 26-Aug-2020Document1 pageScan 26-Aug-2020chhaayaachitran akshuNo ratings yet
- 12 Aldehydes Ketones and Carboxylic AcidsDocument2 pages12 Aldehydes Ketones and Carboxylic AcidsPrasannaNo ratings yet
- AldehydeDocument8 pagesAldehydecbs123abcNo ratings yet
- Carbanions II312Document43 pagesCarbanions II312ERMIN RISKIANINo ratings yet
- Pls. Open in Desktop App in Word and Change The View To Web Layout Carboxylic AcidsDocument5 pagesPls. Open in Desktop App in Word and Change The View To Web Layout Carboxylic AcidsFreya SawNo ratings yet
- Carboxylic Acids:: R-Cooh, R-Co HDocument43 pagesCarboxylic Acids:: R-Cooh, R-Co HmacybnzNo ratings yet
- Alcohols, Phenols and EtherDocument7 pagesAlcohols, Phenols and EtherKhatijaa BeeNo ratings yet
- Chapter 5Document38 pagesChapter 5locvo2k3No ratings yet
- PH-6 - Mains - Answers - ChemistryDocument17 pagesPH-6 - Mains - Answers - Chemistrytanu15048No ratings yet
- Carboxylic Acids and Their DerivativeDocument43 pagesCarboxylic Acids and Their DerivativenathasyaNo ratings yet
- Organic Problem EM-1Document10 pagesOrganic Problem EM-1seetharaman8341100% (1)
- Exp't 81: Synthesis of N-Butyl Acetate Via EsterificationDocument8 pagesExp't 81: Synthesis of N-Butyl Acetate Via EsterificationMuhammad Arif TaufiqNo ratings yet
- 6carboxylic Acids PDFDocument29 pages6carboxylic Acids PDFsharmimiameerasanadyNo ratings yet
- Aldehide Şi Cetone FenoliceDocument9 pagesAldehide Şi Cetone FenoliceMarinelaNo ratings yet
- EstersDocument18 pagesEstersArika FindlayNo ratings yet
- (Lec 1 Part 1) AlkanesDocument29 pages(Lec 1 Part 1) AlkanesAyten FawzyNo ratings yet
- Na I I H I C: Reactions and Preparations of Aldehydes and KetonesDocument5 pagesNa I I H I C: Reactions and Preparations of Aldehydes and KetonesJAN JERICHO MENTOYNo ratings yet
- Matriculation Chemistry (Amino Acids) Part 2Document10 pagesMatriculation Chemistry (Amino Acids) Part 2ridwanNo ratings yet
- CC One ShotDocument29 pagesCC One Shotbobbytext8904No ratings yet
- Preparation of Carboxylic AcidsDocument2 pagesPreparation of Carboxylic Acidsudhayadeepak60No ratings yet
- Ald&Ketone IDocument41 pagesAld&Ketone IHarsha Y MNo ratings yet
- Aldehydes, Ketones and Carboxylic Acids: Chapter-12Document37 pagesAldehydes, Ketones and Carboxylic Acids: Chapter-12ExodusNo ratings yet
- 27 Alcohol Phenol Ether Formula Sheets Getmarks AppDocument15 pages27 Alcohol Phenol Ether Formula Sheets Getmarks AppFLASH FFNo ratings yet
- 5 Aldehydes and Ketones-Structure and PreparationDocument41 pages5 Aldehydes and Ketones-Structure and PreparationKeshav JoshiNo ratings yet
- Aldehyde and KetonesDocument41 pagesAldehyde and KetonesJerome DimaanoNo ratings yet
- Aldehydes and KetonesDocument41 pagesAldehydes and KetonesJerome DimaanoNo ratings yet
- Aldehydes Ketones and Carboxylic AcidsDocument3 pagesAldehydes Ketones and Carboxylic Acidsbalaganesh1505No ratings yet
- CH CooDocument2 pagesCH CooPawan SharmaNo ratings yet
- Name Reactions Organic 12Document15 pagesName Reactions Organic 12Ronak VarshneyNo ratings yet
- Alcohols Phenols and EthersDocument3 pagesAlcohols Phenols and EthersSubath KumarNo ratings yet
- Cbse Test Paper-03 CLASS - XII CHEMISTRY (Aldehydes, Ketones and Carboxylic Acids) (Answer) Topic:-ConversionsDocument2 pagesCbse Test Paper-03 CLASS - XII CHEMISTRY (Aldehydes, Ketones and Carboxylic Acids) (Answer) Topic:-ConversionsShreyash KolekarNo ratings yet
- Carboxylic Acids DerivativesDocument18 pagesCarboxylic Acids DerivativesshyroneruttoNo ratings yet
- Đ Xu T Cơ CH Cho Các PH N NG: Check BoxDocument22 pagesĐ Xu T Cơ CH Cho Các PH N NG: Check BoxStormy StudiosNo ratings yet
- TutorialDocument27 pagesTutorialSiti NuraqidahNo ratings yet
- Nucleophilic Reactions Involving Enolate AnionsDocument44 pagesNucleophilic Reactions Involving Enolate AnionsRia SafitriNo ratings yet
- EAMCET QR Chemistry SR Chem 17.organic Chemistry Carboxylic AcidsDocument5 pagesEAMCET QR Chemistry SR Chem 17.organic Chemistry Carboxylic AcidsJagadeesh Goli100% (2)
- Chemistry Online NotesDocument16 pagesChemistry Online NotesBharti YadavNo ratings yet
- Alcohol Phenols and EthersDocument11 pagesAlcohol Phenols and Ethersvinay368kNo ratings yet
- 1 s2.0 S0040403900879137 MainDocument4 pages1 s2.0 S0040403900879137 MainCabNo ratings yet
- Asam Karboksilat 5Document13 pagesAsam Karboksilat 5Faykar RezaNo ratings yet
- 2021 Problemario QOHBDocument6 pages2021 Problemario QOHBRebeca VegaNo ratings yet
- EstersDocument22 pagesEstersSania KhanNo ratings yet
- DGT Organic Compounds C NitrogenDocument15 pagesDGT Organic Compounds C Nitrogensc5753972No ratings yet
- UreacycleDocument18 pagesUreacycleChudasama DhruvrajsinhNo ratings yet
- Organic Molecules React in Predictable Ways: Functional GroupsDocument21 pagesOrganic Molecules React in Predictable Ways: Functional GroupsNia Rakhmayanti NurdinNo ratings yet
- Chapter 10 Efliza 2021Document37 pagesChapter 10 Efliza 2021NURIN SOFIYA BT ZAKARIA / UPMNo ratings yet
- Aldol Reaction - ChemistryDocument7 pagesAldol Reaction - ChemistryGamer HelperNo ratings yet
- Common Names of Organic CompoundsDocument10 pagesCommon Names of Organic CompoundsGear Less Gamer64% (14)
- Aldehydes Notes 27 May 2023Document7 pagesAldehydes Notes 27 May 2023Aafia AlamNo ratings yet
- Che 176 AlkanolsDocument42 pagesChe 176 Alkanolsodunowo usmanNo ratings yet
- 202003291608409347arun Sethi SteroidDocument7 pages202003291608409347arun Sethi SteroidVishva AegonNo ratings yet
- Organic Chemistry Test-1 On Total Syllabus: Single CorrectDocument5 pagesOrganic Chemistry Test-1 On Total Syllabus: Single CorrectVanshaj GuptaNo ratings yet
- Chem f4 NotesDocument206 pagesChem f4 Notesjacob naibeiNo ratings yet
- Classes of Organic CompoundsDocument2 pagesClasses of Organic CompoundsKatreng VasquezNo ratings yet
- 7.aldehydes and KetonesExerciseDocument60 pages7.aldehydes and KetonesExerciseDEVIKAA ARUNNo ratings yet
- Aep - CPP - 2Document11 pagesAep - CPP - 2ayesha sheikhNo ratings yet
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- India's Contribution To The Universal Declaration of Human RightsDocument34 pagesIndia's Contribution To The Universal Declaration of Human Rightschhaayaachitran akshuNo ratings yet
- Course Title: Legislative Drafting Course Code: LAW 431 Credit Units: 3 Level: UG L T P/ S SW/F W Total Credit UnitsDocument6 pagesCourse Title: Legislative Drafting Course Code: LAW 431 Credit Units: 3 Level: UG L T P/ S SW/F W Total Credit Unitschhaayaachitran akshuNo ratings yet
- Security Council and Main Organs of UNDocument2 pagesSecurity Council and Main Organs of UNchhaayaachitran akshuNo ratings yet
- French Booklet ViiDocument52 pagesFrench Booklet Viichhaayaachitran akshuNo ratings yet
- Case Summary: FactsDocument3 pagesCase Summary: Factschhaayaachitran akshuNo ratings yet
- Relationship Which Exists Amongst and Between Water, Air and Land, and Human Beings, Other Living Creatures, Plants, Micro-Organism and PropertyDocument2 pagesRelationship Which Exists Amongst and Between Water, Air and Land, and Human Beings, Other Living Creatures, Plants, Micro-Organism and Propertychhaayaachitran akshuNo ratings yet
- Format For Course Curriculum: Uttar PradeshDocument6 pagesFormat For Course Curriculum: Uttar Pradeshchhaayaachitran akshuNo ratings yet
- Lectures On Code of Civil Procedure: by Ashutosh Kumar Shukla Asst - Prof. Amity Law SchoolDocument9 pagesLectures On Code of Civil Procedure: by Ashutosh Kumar Shukla Asst - Prof. Amity Law Schoolchhaayaachitran akshuNo ratings yet
- MCQ AnswersDocument1 pageMCQ Answerschhaayaachitran akshuNo ratings yet
- Amity Law School Lucknow Campus: TOPIC:-What Relevance Does Zero FIR Have?Document10 pagesAmity Law School Lucknow Campus: TOPIC:-What Relevance Does Zero FIR Have?chhaayaachitran akshuNo ratings yet
- Repeal of StatutesDocument10 pagesRepeal of Statuteschhaayaachitran akshuNo ratings yet
- AnswerDocument2 pagesAnswerchhaayaachitran akshuNo ratings yet
- Growth of Trade UnionDocument11 pagesGrowth of Trade Unionchhaayaachitran akshuNo ratings yet
- Indian Evidence ACT 1872: Relevancy & AdmissibilityDocument9 pagesIndian Evidence ACT 1872: Relevancy & Admissibilitychhaayaachitran akshu100% (1)
- Aand Povenes: O Upendro NalhDocument8 pagesAand Povenes: O Upendro Nalhchhaayaachitran akshuNo ratings yet
- Case Study On SanskritisationDocument8 pagesCase Study On Sanskritisationchhaayaachitran akshuNo ratings yet
- Labour Law PresentationDocument7 pagesLabour Law Presentationchhaayaachitran akshuNo ratings yet
- Indian Evidence ACT 1872: SubtitleDocument12 pagesIndian Evidence ACT 1872: Subtitlechhaayaachitran akshuNo ratings yet
- Laporan Harian Sub LPLPO Unit Layanan Puskesmas TamalateDocument96 pagesLaporan Harian Sub LPLPO Unit Layanan Puskesmas Tamalatepkm tamalateNo ratings yet
- Chemistry Test ss3Document3 pagesChemistry Test ss3lawaljamiuadebayoNo ratings yet
- Alkanes, Alkenes, Alkynes and Cyclic Hydrocarbons WorksheetDocument5 pagesAlkanes, Alkenes, Alkynes and Cyclic Hydrocarbons WorksheetZhang MaxwellNo ratings yet
- Nfpa 497 2012Document1 pageNfpa 497 2012Kaka Baba100% (1)
- Amines WordDocument25 pagesAmines Wordnvmohankumar85No ratings yet
- FArmasi RS Dan Klinik KomprehensifDocument8 pagesFArmasi RS Dan Klinik Komprehensifklinik dktNo ratings yet
- 3 O-RINGS 0,90X0,90 Silicone 4 O-RINGS 1,07X1,27 SiliconeDocument161 pages3 O-RINGS 0,90X0,90 Silicone 4 O-RINGS 1,07X1,27 SiliconetmsxptoNo ratings yet
- Worksheet: Subject: Batch: Date of Issue: Unit: ID No.: Due DateDocument4 pagesWorksheet: Subject: Batch: Date of Issue: Unit: ID No.: Due Datedharmendra gaikwadNo ratings yet
- Perhitungan C-Per ProteinDocument3 pagesPerhitungan C-Per ProteinMasref21No ratings yet
- Amino Acids BiochemistryDocument32 pagesAmino Acids BiochemistryKavita MahaseNo ratings yet
- Class - XII Class - XII: (Aryl Halides)Document30 pagesClass - XII Class - XII: (Aryl Halides)Utshav paudelNo ratings yet
- Organic ChemistryDocument38 pagesOrganic ChemistryJackie DidaNo ratings yet
- Activity 12 The HydrocarbonsDocument1 pageActivity 12 The HydrocarbonsWENDEL MAYORNo ratings yet
- Tata Nama Alcohols and EthersDocument4 pagesTata Nama Alcohols and EthersAde RakhaNo ratings yet
- Summary of IUPAC Nomenclature of Organic CompoundsDocument9 pagesSummary of IUPAC Nomenclature of Organic Compoundsstreetcribdealer100% (1)
- Rencana Distribusi Obat PuskesmasDocument58 pagesRencana Distribusi Obat PuskesmasLita Rahma YulitaNo ratings yet
- Name Pi R Group: Characteristic Three LetterDocument1 pageName Pi R Group: Characteristic Three LetterAloysius QuitaligNo ratings yet
- Home Assignment-4Document66 pagesHome Assignment-4ansh guptaNo ratings yet
- BP202TP PDFDocument2 pagesBP202TP PDFVINOD CHOUDHARYNo ratings yet
- CHCHCHBR X Y Z: Cbse Test Paper-04 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes)Document1 pageCHCHCHBR X Y Z: Cbse Test Paper-04 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes)Shreyash KolekarNo ratings yet
- CBSE Class 12 Chem Notes Question Bank Amines PDFDocument21 pagesCBSE Class 12 Chem Notes Question Bank Amines PDFMr bhupendra Singh rathoreNo ratings yet
- Industrial Manufactured:: AmmoniaDocument9 pagesIndustrial Manufactured:: AmmoniaFidree AzizNo ratings yet
- Batch Topic DPPDocument7 pagesBatch Topic DPPSiddharth ShahNo ratings yet
- Kepmenkes 659-2017 Formularium NasionalDocument45 pagesKepmenkes 659-2017 Formularium NasionalMarhamah AmahNo ratings yet
- Halogenalkanes Elimination and OzoneDocument88 pagesHalogenalkanes Elimination and Ozone22S48 SUNDARAM RAMASUBBU RAKSHANo ratings yet
- Alkenes and Alkynes-StudentsDocument25 pagesAlkenes and Alkynes-StudentsTie Teck Hoe100% (1)
- LIPID MAPS Lipid Chemistry Tutorial: San Diego Supercomputer Center University of California, San DiegoDocument66 pagesLIPID MAPS Lipid Chemistry Tutorial: San Diego Supercomputer Center University of California, San Diegoputu wijayantiNo ratings yet
- Speciality Chemicals Product ListDocument201 pagesSpeciality Chemicals Product ListJakin RookNo ratings yet
- 11 Worksheet HydrocarbonDocument2 pages11 Worksheet HydrocarbonAakif RazaNo ratings yet
- Price Quotation 81419Document24 pagesPrice Quotation 81419Joshua Kevin Carl Tabar-GolpeNo ratings yet