Professional Documents
Culture Documents
Eyrthrokinetics
Uploaded by
Kenneth Jake BatiduanCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Eyrthrokinetics
Uploaded by
Kenneth Jake BatiduanCopyright:
Available Formats
ERYTHROKINETICS their cells does not unload oxygen to the tissues
readily, so newborns are slightly hypoxic compared
• Erythrokinetics is the term describing the dynamics
with adults. To compensate, they make more RBCs.
of RBC production and destruction.
• Erythron is the name given to the collection of all •
stages of erythrocytes throughout the body: the
developing precursors in the bone marrow and the • Hypoxia increases EPO production in peritubular
circulating erythrocytes in the peripheral blood and cells mainly by transcriptional regulation. The EPO
the vascular spaces within specific organs such as gene has a hypoxia-sensitive region (enhancer) in
the spleen. When the term erythron is used, it
conveys the concept of a unified functional tissue. its 3′ regulatory component.15 When oxygen tension
in the cell decreases, hypoxia-inducible factor-1, a
*The erythron is distinguished from the RBC mass. The transcription factor, is assembled in the
erythron is the entirety of erythroid cells in the body,
whereas the RBC mass refers only to the cells in cytoplasm,16 migrates to the nucleus, and interacts
circulation. with the 3′ enhancer of the gene. This results in
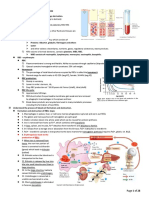
Hypoxia—the stimulus to red blood cell production transcription of more EPO messenger RNA
molecules, and production of more EPO.
• As mentioned previously, the role of RBCs is to carry
oxygen. To regulate the production of RBCs for that Erythropoietin
purpose, the body requires a mechanism for sensing
whether there is adequate oxygen being carried to Structure.
the tissues. If not, RBC production and the functional
efficiency of existing cells must be enhanced. Thus a EPO is a thermostable, nondialyzable, glycoprotein
second feature of the oxygen-sensing system must hormone with a molecular weight of 34 kD.17 It consists of
be a mechanism for influencing the production of a carbohydrate unit that reacts specifically with RBC
RBCs. receptors and a terminal sialic acid unit, which is
• The primary oxygen-sensing system of the body is necessary for biological activity in vivo.18 On desialation,
located in peritubular fibroblasts of the EPO activity ceases.1
kidney. Hypoxia, too little tissue oxygen, is detected
by the peritubular cells, which Action.
produce erythropoietin (EPO), the major stimulatory
cytokine for RBCs. Under normal circumstances, the
amount of EPO produced fluctuates very little, EPO is a true hormone, being produced at one location
maintaining a level of RBC production that is (the kidney) and acting at a distant location (the bone
sufficient to replace the approximately 1% of RBCs marrow). It is a growth factor (or cytokine) that initiates an
that normally die each day. When there is intracellular message to the developing RBCs; this
hemorrhage, increased RBC destruction, or other
factors that diminish the oxygen-carrying capacity of process is called signal transduction. EPO must bind to
the blood, the production of EPO is increased. its receptor on the surface of cells to initiate the signal or
message. The receptor is a transmembrane homodimer
consisting of two identical polypeptide chains.20 EPO-
Hypoxia and Red Blood Cell Production responsive cells vary in their sensitivity to EPO.21 Some
• Decreased RBC number, however, is only one are able to respond to low levels of EPO,22 whereas
cause of hypoxia. Another cause is the failure of others require higher levels. In healthy circumstances
each RBC to carry as much oxygen as it should. when RBC production needs to proceed at a modest but
This can occur because the hemoglobin is defective regular rate, the cells requiring only low levels of EPO
or because there is not enough hemoglobin in each respond. If EPO levels rise secondary to hypoxia,
cell. The hypoxia may be unrelated to the RBCs in however, a larger population of EPO-sensitive cells is
any way; poor lung function resulting in diminished able to respond.
oxygenation of existing RBCs is an example.
• The binding of EPO, the ligand, to its receptor on
• The kidney’s hypoxia sensor cannot know why there erythrocyte progenitors initiates a cascade of
is hypoxia, but it does not matter. Even when there intracellular events (“the program”) that ultimately
are plenty of RBCs compared with the reference leads to cell division, maturation, and more red
interval, if there is still hypoxia, stimulation of RBC blood cells entering the circulation. EPO’s effects are
production is warranted because the numbers mediated by Janus-activated tyrosine kinase 2
present are not meeting the oxygen need. An (JAK2) signal transducers that are associated with
elevation of RBC numbers above the reference the cytoplasmic domain of the EPO receptor and
interval, erythrocytosis, is seen in conditions such as ultimately affect gene expression in the RBC
lung disease and cardiac disease in which the blood nucleus. 23 EPO has three major effects: allowing
is not being well oxygenated. Newborns have higher early release of reticulocytes from the bone marrow,
numbers of RBCs because the fetal hemoglobin in preventing apoptotic cell death, and reducing the
time needed for cells to mature in the bone marrow. by apoptosis, and it is part of the cell’s genetic
The essence is that EPO puts more RBCs into the program.
circulation at a faster rate than occurs without its Process of apoptosis.
stimulation.
• Apoptosis is a sequential process characterized by,
Early release of reticulocytes. among other things, the degradation of chromatin
• EPO promotes early release of developing erythroid into fragments of varying size that are multiples of
precursors from the marrow by two mechanisms. 180 to 185 base pairs long; protein clustering; and
EPO induces changes in the adventitial cell layer of activation of transglutamase. This is in contrast to
the marrow/sinus barrier that increase the width of necrosis, in which cell injury causes swelling and
the spaces for RBC egress into the sinus.24 This
mechanism alone, however, is insufficient for cells to lysing with release of cytoplasmic contents that
leave the marrow. RBCs are held in the marrow stimulate an inflammatory response (Chapter 6).
because they express surface membrane receptors Apoptosis is not associated with inflammation.
for adhesive molecules located on the bone marrow
stroma. EPO downregulates the expression of these
receptors so that cells can exit the marrow earlier
than they normally would. The result is the presence • During the sequential process of apoptosis, the
in the circulation of reticulocytes that are still very following morphologic changes can be seen:
basophilic because they have not spent as much condensation of the nucleus, causing increased
time degrading their ribosomes or making basophilic staining of the chromatin; nucleolar
hemoglobin as they normally would before entering disintegration; and shrinkage of cell volume with
the bloodstream. These are called shift
reticulocytes because they have been shifted from concomitant increase in cell density and compaction
the bone marrow early (Figure 8-8, A). Their bluish of cytoplasmic organelles, while mitochondria remain
cytoplasm with Wright stain is evident, so the overall normal.28 This is followed by a partition of cytoplasm
blood picture is said to have polychromasia. Even and nucleus into membrane-bound apoptotic bodies
nucleated RBCs (i.e., normoblasts) can be released that contain varying amounts of ribosomes,
early in cases of extreme anemia when the demand organelles, and nuclear material. The last stage of
for RBCs in the peripheral circulation is great. degradation produces nuclear DNA fragments
Releasing cells from the marrow early is a quick fix, consisting of multimers of 180 to 185 base pair
so to speak; it is limited in effectiveness because the
available precursors in the marrow are depleted segments. Characteristic blebbing of the plasma
within several days and still may not be enough to membrane is observed. The apoptotic cell contents
meet the need in the peripheral blood for more cells. remain membrane-bound and are ingested by
A more sustained response is required in times of macrophages, which prevents an inflammatory
increased need for RBCs in the circulation. reaction. The membrane-bound vesicles display so-
Inhibition of apoptosis. called “eat me” signals on the membrane surface
(discussed later) that promote macrophage
• A second, and probably more important, mechanism ingestion.29
by which EPO increases the number of circulating
Evasion of apoptosis by erythroid progenitors and
RBCs is by increasing the number of cells that will
be able to mature into circulating erythrocytes. It precursors.
does this by decreasing apoptosis, the programmed
death of RBC progenitors. • One effect of EPO is an indirect avoidance of
apoptosis by removing an apoptosis induction
Apoptosis: Programmed cell death. signal. Apoptosis of RBCs is a cellular process that
depends on a signal from either the inside or outside
• As noted previously, it takes about 18 to 21 days to of the cell. Among the crucial molecules in the
produce an RBC from stimulation of the earliest external messaging system is the death receptor
erythroid progenitor (BFU-E) to release from the Fas on the membrane of the earliest RBC
bone marrow. In times of increased need for RBCs, precursors, while its ligand, FasL, is expressed by
such as when there is loss from the circulation more mature RBCs. When EPO levels are low, cell
during hemorrhage, this time lag would be a production should be at a low rate because hypoxia
significant problem. One way to prepare for such a is not present. The excess early erythroid precursors
need would be to maintain a store of mature RBCs should undergo apoptosis. This occurs when the
in the body for emergencies. RBCs cannot be stored older FasL-bearing erythroid precursors, such as
in the body for this sort of eventuality, however, polychromatic normoblasts, cross-link with Fas-
because they have a limited life span. Therefore, marked immature erythroid precursors, such as
instead of storing mature cells for emergencies, the pronormoblasts and basophilic normoblasts, which
body produces more CFU-Es than needed at all are then stimulated to undergo apoptosis.28 As long
times. When there is a basal or steady-state as the more mature cells with FasL are present in
demand for RBCs, the extra progenitors are allowed the marrow, erythropoiesis is subdued. If the FasL-
to die. When there is an increased demand for bearing cells are depleted, as when EPO stimulates
RBCs, however, the RBC progenitors have about an early marrow release, the younger Fas-positive
8- to 10-day head start in the production process. precursors are allowed to develop, which increases
This process of intentional wastage of cells occurs the overall output of RBCs from the marrow. Thus
early release of older cells in response to EPO example reference interval is 4 to 27 mU/
indirectly allows more of the younger cells to mature. L.40 Increased amounts of EPO in the urine are
expected in most patients with anemia, with the
exception of patients with anemia caused by renal
• A second mechanism for escaping apoptosis exists
disease.
for RBC progenitors: direct EPO rescue from
apoptosis. This is the major way in which EPO is Therapeutic uses of erythropoietin.
able to increase RBC production. When EPO binds
to its receptor on the CFU-E, one of the effects is to • Recombinant erythropoietin is used as therapy in
certain anemias such as those associated with
reduce production of Fas ligand.31 Thus the younger chronic kidney disease and chemotherapy. It is also
cells avoid the apoptotic signal from the older cells. used to stimulate RBC production prior to
Additionally, EPO is able to stimulate production of autologous blood donation and after bone marrow
transplantation.
various anti-apoptotic molecules, which allows the
cell to survive and mature.31, 32 The cell that has the • Unfortunately, some athletes illicitly use EPO
most EPO receptors and is most sensitive to EPO injections to increase the oxygen-carrying capacity
of their blood to enhance endurance and stamina,
rescue is the CFU-E, although the late BFU-E and especially in long-distance running and cycling. The
early pronormoblast have some receptors.33 Without use of EPO is one of the methods of blood doping,
EPO, the CFU-E does not survive.34 and aside from being banned in organized sports
events, it increases the RBC count and blood
Reduced marrow transit time. viscosity to dangerously high levels and can lead to
fatal arterial and venous thrombosis
• Apoptosis rescue is the major way in which EPO increases Other stimuli to erythropoiesis
RBC mass—by increasing the number of erythroid cells that • In addition to tissue hypoxia, other factors influence
survive and mature to enter the circulation. Another effect of RBC production to a modest extent. It is well
documented that testosterone directly stimulates
EPO is to increase the rate at which the surviving precursors
erythropoiesis, which partially explains the higher
can enter the circulation. This is accomplished by two hemoglobin concentration in men than in women.
means: increased rate of cellular processes and decreased Also, pituitary42 and thyroid43 hormones have been
shown to affect the production of EPO and so have
cell cycle times.
indirect effects on erythropoiesis.
• Among the processes that are accelerated is Erythrocyte destruction
hemoglobin production. As mentioned earlier,
another accelerated process is bone marrow egress • All cells experience the deterioration of their
enzymes over time due to natural catabolism. Most
with the loss of adhesive receptors and the
cells are able to replenish needed enzymes and
acquisition of egress-promoting surface molecules. continue their cellular processes. As a nonnucleated
The other process that is accelerated is the cell, however, the mature erythrocyte is unable to
cessation of division. Cell division takes time and generate new proteins, such as enzymes, so as its
would delay entry of cells to the circulation, so cells cellular functions decline, the cell ultimately
enter cell cycle arrest sooner. As a result, the cells approaches death. The average RBC has sufficient
spend less time maturing in the marrow. enzyme function to live 120 days. Because RBCs
lack mitochondria, they rely on glycolysis for
• EPO also can reduce the time it takes for cells to production of adenosine triphosphate (ATP). The
mature in the marrow by reducing individual cell loss of glycolytic enzymes is central to this process
cycle time, specifically the length of time that cells of cellular aging, called senescence, which
spend between mitoses. culminates in phagocytosis by macrophages. This is
• With the decreased cell cycle time and fewer mitotic the major way in which RBCs die normally.
divisions, the time it takes from pronormoblast to Macrophage-mediated hemolysis (extravascular
reticulocyte can be shortened by about 2 days total. hemolysis)
If the reticulocyte leaves the marrow early, another
day can be saved, and the typical 6-day transit time • At any given time, a substantial volume of blood is in
is reduced to fewer than 4 days under the influence the spleen, which generates an environment that is
of increased EPO. inherently stressful on cells. Movement through the
Measurement of erythropoietin. red pulp is sluggish. The available glucose in the
surrounding plasma is depleted quickly as the cell
• Quantitative measurements of EPO are performed flow stagnates, so glycolysis slows. The pH is low,
on plasma and other body fluids. EPO can be which promotes iron oxidation. Maintaining reduced
measured by chemiluminescence. Although the iron is an energy-dependent process, so factors that
reference interval for each laboratory varies, an promote iron oxidation cause the RBC to expend
more energy and accelerate the catabolism of Mechanical hemolysis (fragmentation or intravascular
enzymes. hemolysis)
• In this hostile environment, aged RBCs succumb to
the various stresses. Their deteriorating glycolytic
processes lead to reduced ATP production, which is
• Although most natural RBC deaths occur in the
spleen, a small portion of RBCs
complicated further by diminished amounts of
available glucose. The membrane systems that rely rupture intravascularly (within the lumen of blood
on ATP begin to fail. Among these are enzymes that vessels). The vascular system can be traumatic to
maintain the location and reduction of phospholipids RBCs, with turbulence occurring in the chambers of
of the membrane. Lack of ATP leads to oxidation of the heart or at points of bifurcation of vessels. Small
membrane lipids and proteins. Other ATP-dependent
enzymes are responsible for maintaining the high breaks in blood vessels and resulting clots can also
level of intracellular potassium while pumping trap and rupture cells. The intravascular rupture of
sodium out of the cells. As this system fails, RBCs from purely mechanical or traumatic stress
intracellular sodium increases and potassium results in fragmentation and release of the cell
decreases. The effect is that the selective
permeability of the membrane is lost and water contents into the plasma; this is
enters the cell. The discoid shape is lost and the cell called fragmentation or intravascular hemolysis.
becomes a sphere
• RBCs must remain highly flexible to exit the spleen • When the membrane of the RBC has been
breached, regardless of where the cell is located
by squeezing through the so-called splenic
when it happens, the cell contents enter the
sieve formed by the endothelial cells lining the
surrounding plasma. Although mechanical lysis is a
venous sinuses and the basement membrane.
relatively small contributor to RBC demise under
Spherical RBCs are rigid and are not able to
normal circumstances, the body still has a system of
squeeze through the narrow spaces; they become
plasma proteins, including haptoglobin and
trapped against the endothelial cells and basement
hemopexin, to salvage the released hemoglobin so
membrane. In this situation, they are readily
that its iron is not lost in the urine.
ingested by macrophages that patrol along the
sinusoidal lining
• Some researchers view erythrocyte death as a
nonnucleated cell version of apoptosis,
termed eryptosis,49 that is precipitated by oxidative
stress, energy depletion, and other mechanisms that
create membrane signals that stimulate
phagocytosis. It is highly likely that there is no single
signal but rather that macrophages recognize
several.
• When an RBC lyses within a macrophage, the major
components are catabolized. The iron is removed
from the heme. It can be stored in the macrophage
as ferritin until transported out. The globin of
hemoglobin is degraded and returned to the
metabolic amino acid pool. The protoporphyrin
component of heme is degraded through several
intermediaries to bilirubin, which is released into the
plasma and ultimately excreted by the liver in bile.
You might also like
- NSG 330 NCLEX Kaplan Med Surg 2 Final Exam Predictor TestDocument18 pagesNSG 330 NCLEX Kaplan Med Surg 2 Final Exam Predictor TestabbieNo ratings yet
- Project Investigatory BiologyDocument24 pagesProject Investigatory BiologyVVM. S.4669100% (2)
- Endterm Week 2 HistotechDocument7 pagesEndterm Week 2 HistotechKenneth Jake BatiduanNo ratings yet
- Borea, P. A., Varani, K., Gessi, S., Merighi, S., & Vincenzi, F. (Eds.) - (2018) - The Adenosine Receptors PDFDocument603 pagesBorea, P. A., Varani, K., Gessi, S., Merighi, S., & Vincenzi, F. (Eds.) - (2018) - The Adenosine Receptors PDFBreiner PeñarandaNo ratings yet
- Blood: Erythropoeisis by U.SivakumarDocument41 pagesBlood: Erythropoeisis by U.SivakumarAkash JaatNo ratings yet
- Unit Viii: The Hematologic SystemDocument1 pageUnit Viii: The Hematologic SystemAqi RamenaiNo ratings yet
- Body Fluids and Blood 2 - 095437Document103 pagesBody Fluids and Blood 2 - 095437TasleemNo ratings yet
- Red Cell.1Document25 pagesRed Cell.1Jude ChinecheremNo ratings yet
- Chapter Blood: RBC Platelet HemostasisDocument89 pagesChapter Blood: RBC Platelet Hemostasisapi-19916399100% (1)
- RBC Production and DestructionDocument44 pagesRBC Production and DestructionNehemiah FranciscoNo ratings yet
- ErytHroCyte ProduCtionDocument8 pagesErytHroCyte ProduCtionMichelle CaamicNo ratings yet
- Physiology Summary Chapter 32Document6 pagesPhysiology Summary Chapter 32gail018No ratings yet
- Red Blood Cells, Anemia, PolycythemiaDocument53 pagesRed Blood Cells, Anemia, PolycythemiaDayen Lim100% (1)
- Hematology 1 L3 Erythrocytes LectureDocument3 pagesHematology 1 L3 Erythrocytes LectureChelze Faith DizonNo ratings yet
- הגישה לאנמיהDocument9 pagesהגישה לאנמיהddNo ratings yet
- Ap2 Chapter18 Powerpoint Blood SU21Document37 pagesAp2 Chapter18 Powerpoint Blood SU21btheresakNo ratings yet
- Circulatory System Part I - BloodDocument123 pagesCirculatory System Part I - BloodAngelyn DomingoNo ratings yet
- Physiology Lecture 2: RBC S Characteristics, Function and FormationDocument20 pagesPhysiology Lecture 2: RBC S Characteristics, Function and FormationdarkboiNo ratings yet
- Physiology of Blood 1Document66 pagesPhysiology of Blood 1Hiruni FernandoNo ratings yet
- RBC Formation, Anemia, WBC FunctionDocument28 pagesRBC Formation, Anemia, WBC FunctionlifecostNo ratings yet
- Haematopoiesis and Red Blood Cells 2013 Surgery OxfordDocument6 pagesHaematopoiesis and Red Blood Cells 2013 Surgery OxfordSanjaya SenevirathneNo ratings yet
- BLOOD Physiology Guyton 14th Edition Chapter 33Document57 pagesBLOOD Physiology Guyton 14th Edition Chapter 33Dr Alamzeb Jadoon100% (1)
- Alangui, Hannah Vannerie A. Module 7 Lec ActDocument6 pagesAlangui, Hannah Vannerie A. Module 7 Lec ActHannah Vannerie AlanguiNo ratings yet
- 1 Red Blood Cells Anemia and PolycythemiaDocument43 pages1 Red Blood Cells Anemia and PolycythemiaGeevee Naganag VentulaNo ratings yet
- Study Guide Exam 1Document4 pagesStudy Guide Exam 1NicolleNo ratings yet
- Red Blood Cells: Wednesday, Dece Mber 08, 2021 Presented by U A.AlboraiDocument36 pagesRed Blood Cells: Wednesday, Dece Mber 08, 2021 Presented by U A.Alboraiborai8No ratings yet
- Anhydrase, An Enzyme That Catalyzes TheDocument15 pagesAnhydrase, An Enzyme That Catalyzes TheJohnny eawNo ratings yet
- Erythrocyte Differentiation: Erythropoiesis Is The Process by WhichDocument2 pagesErythrocyte Differentiation: Erythropoiesis Is The Process by Which:)No ratings yet
- Feline Nonregenerative Anemia Pathophysiology and ErilologiesDocument7 pagesFeline Nonregenerative Anemia Pathophysiology and ErilologiesTORRES GUTIERREZ JOYNY RAQUEL catedraticoNo ratings yet
- LEC 5 RBC METABOLISM AND Membrane StructureDocument30 pagesLEC 5 RBC METABOLISM AND Membrane StructureFrancis ValdezNo ratings yet
- 1 s2.0 S0301472X08003883 MainDocument12 pages1 s2.0 S0301472X08003883 MainHồ Đại NghĩaNo ratings yet
- CH 19 - The Blood - SV Complete (1) Kin 267 Module 7Document53 pagesCH 19 - The Blood - SV Complete (1) Kin 267 Module 7J aNo ratings yet
- Red Blood Cell BiochemistryDocument19 pagesRed Blood Cell BiochemistryPrincewill SeiyefaNo ratings yet
- BloodDocument6 pagesBloodCaroleNo ratings yet
- Chapter 13 highlights key aspects of bloodDocument8 pagesChapter 13 highlights key aspects of bloodDayledaniel Sorveto0% (1)
- Chap 8Document17 pagesChap 8Shane EsparasNo ratings yet
- Lecture7 - ErythropoiesisDocument31 pagesLecture7 - ErythropoiesisMarshina KunhammedNo ratings yet
- Final Nursing Physiology 1 BookDocument46 pagesFinal Nursing Physiology 1 BooklolNo ratings yet
- 1 Blood22Document48 pages1 Blood22Wizz Háķìm ĻêşòwNo ratings yet
- AnemiaDocument47 pagesAnemiaAjay KumarNo ratings yet
- 3A Blood PhysiologyDocument7 pages3A Blood PhysiologyRen AlvNo ratings yet
- Physiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaDocument60 pagesPhysiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaZidaneNo ratings yet
- Aspek Biokimiawi HematologiDocument72 pagesAspek Biokimiawi HematologiExcelsis Deo SombolinggiNo ratings yet
- Blood: Beenish Gul Khattak Lecturer Pharmacy Abasyn University PeshawarDocument66 pagesBlood: Beenish Gul Khattak Lecturer Pharmacy Abasyn University PeshawarEsperanza KtkNo ratings yet
- Red Blood Cells, Anemia, and PolycythemiaDocument7 pagesRed Blood Cells, Anemia, and PolycythemiaShi no Me100% (1)
- Erythropoiesis and ErythropoietinDocument29 pagesErythropoiesis and ErythropoietinmaduksjaycryptoNo ratings yet
- Hematology L7_٠٨٢١١٩Document22 pagesHematology L7_٠٨٢١١٩shakib33001No ratings yet
- Topics of This Lecture: RBC: - Structural Characteristics - Hemoglobin - Erythropoiesis - Erythrocytes DestructionDocument23 pagesTopics of This Lecture: RBC: - Structural Characteristics - Hemoglobin - Erythropoiesis - Erythrocytes DestructionHashim GhazoNo ratings yet
- Blood (Notes)Document12 pagesBlood (Notes)Angel Rose BrillanteNo ratings yet
- Physiology Summary Chapter 32Document2 pagesPhysiology Summary Chapter 32gail018No ratings yet
- The Functions and Components of BloodDocument21 pagesThe Functions and Components of BloodShekinah Lynn A. PocdolNo ratings yet
- S7L01 - BloodDocument49 pagesS7L01 - BloodSuad WarsameNo ratings yet
- Physiology - Guyton BLOODDocument6 pagesPhysiology - Guyton BLOODIsra K. SoomroNo ratings yet
- Blood and Its ComponentsDocument8 pagesBlood and Its ComponentsMyat NyanNo ratings yet
- ERYTHROPOISISDocument22 pagesERYTHROPOISISW.F KareemNo ratings yet
- Blood Physiology: Biconcave Discs 120 DaysDocument6 pagesBlood Physiology: Biconcave Discs 120 DaysHanamiNo ratings yet
- Physiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaDocument65 pagesPhysiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaRizal AbdurrahmanNo ratings yet
- Erythrocytes Red Blood CellsDocument54 pagesErythrocytes Red Blood Cellsclarence fernandezNo ratings yet
- Formation of RBCsDocument72 pagesFormation of RBCsSodeinde SimeonNo ratings yet
- Erythropoietin PDFDocument13 pagesErythropoietin PDFZeynep SemenNo ratings yet
- 6 - Blood PhysiologyDocument32 pages6 - Blood PhysiologyRyan NegadNo ratings yet
- Red Blood Cells, Functions, Diseases A Simple Guide To The Condition, Diagnosis, Treatment, And Related ConditionsFrom EverandRed Blood Cells, Functions, Diseases A Simple Guide To The Condition, Diagnosis, Treatment, And Related ConditionsNo ratings yet
- Fast Facts: Leukemia: From initial gene mutation to survivorship supportFrom EverandFast Facts: Leukemia: From initial gene mutation to survivorship supportNo ratings yet
- Fast Facts: Deficiencia de piruvato quinasa para pacientes y familiares: Una enfermedad genética rara que afecta a los glóbulos rojos Información + Asumir el control = El mejor resultadoFrom EverandFast Facts: Deficiencia de piruvato quinasa para pacientes y familiares: Una enfermedad genética rara que afecta a los glóbulos rojos Información + Asumir el control = El mejor resultadoNo ratings yet
- Resume For WorkDocument2 pagesResume For WorkKenneth Jake BatiduanNo ratings yet
- Wearing School UniformsDocument5 pagesWearing School UniformsKenneth Jake Batiduan100% (1)
- Crossmatching (Major and Minor)Document1 pageCrossmatching (Major and Minor)Kenneth Jake BatiduanNo ratings yet
- Kenneth Jake T. BatiduanDocument1 pageKenneth Jake T. BatiduanKenneth Jake BatiduanNo ratings yet
- Application LetterDocument4 pagesApplication LetterKenneth Jake BatiduanNo ratings yet
- 5) Modified Diamond's Medium Was Used For The Culture of T.vaginalisDocument2 pages5) Modified Diamond's Medium Was Used For The Culture of T.vaginalisKenneth Jake BatiduanNo ratings yet
- Chemical Fixatives and Tissue Processing StepsDocument8 pagesChemical Fixatives and Tissue Processing StepsKenneth Jake BatiduanNo ratings yet
- Pneumoniae and Neisseria Species. It Is Also: Preparation of Blood Agar PlateDocument4 pagesPneumoniae and Neisseria Species. It Is Also: Preparation of Blood Agar PlateKenneth Jake BatiduanNo ratings yet
- Preparation of BAP and CHOCDocument5 pagesPreparation of BAP and CHOCKenneth Jake BatiduanNo ratings yet
- Maturation SequenceDocument4 pagesMaturation SequenceKenneth Jake BatiduanNo ratings yet
- Seminal Fluid AnalysisDocument11 pagesSeminal Fluid AnalysisKenneth Jake BatiduanNo ratings yet
- Klebsiella Shigella Yersinia: Have Little Value in IDDocument6 pagesKlebsiella Shigella Yersinia: Have Little Value in IDKenneth Jake Batiduan100% (1)
- Section Cutting Methods and Microtome TypesDocument8 pagesSection Cutting Methods and Microtome TypesKenneth Jake BatiduanNo ratings yet
- Erythrocytes MetabolismDocument20 pagesErythrocytes MetabolismKenneth Jake BatiduanNo ratings yet
- Endterm Week 1 HistopathDocument6 pagesEndterm Week 1 HistopathKenneth Jake BatiduanNo ratings yet
- Normoblastic Maturation: ErythropoiesisDocument2 pagesNormoblastic Maturation: ErythropoiesisKenneth Jake BatiduanNo ratings yet
- Cestodes Summary FinalsDocument2 pagesCestodes Summary FinalsKenneth Jake Batiduan100% (1)
- (Pe 4) Notes On Swimming - MidtermDocument4 pages(Pe 4) Notes On Swimming - MidtermKenneth Jake BatiduanNo ratings yet
- RBC membrane deformability and lipid compositionDocument2 pagesRBC membrane deformability and lipid compositionKenneth Jake BatiduanNo ratings yet
- Have You Ever Experience Non Specific Symptoms Like Epigastric PainDocument1 pageHave You Ever Experience Non Specific Symptoms Like Epigastric PainKenneth Jake BatiduanNo ratings yet
- Student - The Antioxidant - Power - of - Colorful - Fruits - and - VegetablesDocument4 pagesStudent - The Antioxidant - Power - of - Colorful - Fruits - and - VegetablesKenneth Jake BatiduanNo ratings yet
- Primary TissuesDocument1 pagePrimary TissuesKenneth Jake BatiduanNo ratings yet
- Notebook Labels Per SubjectDocument4 pagesNotebook Labels Per SubjectKenneth Jake BatiduanNo ratings yet
- Phil HistDocument11 pagesPhil HistKenneth Jake BatiduanNo ratings yet
- 10 Thinks You Can Do To Prevent Violence (PDF) - 201505121458185398Document1 page10 Thinks You Can Do To Prevent Violence (PDF) - 201505121458185398Kenneth Jake BatiduanNo ratings yet
- 6 Connective TissueDocument1 page6 Connective TissueKenneth Jake BatiduanNo ratings yet
- Case Ana RubcricsDocument2 pagesCase Ana RubcricsKenneth Jake BatiduanNo ratings yet
- Guide Miss GemDocument4 pagesGuide Miss GemKenneth Jake BatiduanNo ratings yet
- Surgical Management of Head InjuryDocument12 pagesSurgical Management of Head InjurySumiethaa ShanmuganathanNo ratings yet
- Multiple Sclerosis of The Cervical SpineDocument6 pagesMultiple Sclerosis of The Cervical SpineAmajida RizkyNo ratings yet
- Central Luzon Doctors' Hospital Educational Institution Anatomy SystemsDocument5 pagesCentral Luzon Doctors' Hospital Educational Institution Anatomy SystemsJenny Sumangil EspejoNo ratings yet
- Sherif EL Hawary, MD Professor of Internal Medicine Kasr AL AiniDocument35 pagesSherif EL Hawary, MD Professor of Internal Medicine Kasr AL Aini670411No ratings yet
- Operations Management Processes and Supply Chains 10th Edition Krajewski Test BankDocument26 pagesOperations Management Processes and Supply Chains 10th Edition Krajewski Test BankRobertBoydeona100% (37)
- 心血管外科术语汉英对照表Document13 pages心血管外科术语汉英对照表Ted TsieNo ratings yet
- The A To Z of The HeartDocument259 pagesThe A To Z of The HeartAspenPharma100% (2)
- Obana ProtocolDocument4 pagesObana Protocolpaiskopaxiew888No ratings yet
- Dr. YSR Aarogyasri Health Care Trust 4Document16 pagesDr. YSR Aarogyasri Health Care Trust 4saiprashanth5.11No ratings yet
- Winkler - Chapter 8. Khalid AlshareefDocument4 pagesWinkler - Chapter 8. Khalid AlshareefKhalid Bin FaisalNo ratings yet
- Piriformis Syndrome ReviewDocument6 pagesPiriformis Syndrome Reviewismael wandikboNo ratings yet
- Cardiovascular Physiology - Achilles Pappano, 10EDocument305 pagesCardiovascular Physiology - Achilles Pappano, 10EPanda Mimi100% (1)
- Adrenal and PheochromocytomaDocument86 pagesAdrenal and Pheochromocytomarajan kumar100% (1)
- Essentials of Medical Language 3rd Edition Allan Test BankDocument143 pagesEssentials of Medical Language 3rd Edition Allan Test BankDebbieCollinsokpzd100% (14)
- Chapter 3 - Tissue Renewal, Regeneration, and RepairDocument12 pagesChapter 3 - Tissue Renewal, Regeneration, and RepairAgnieszka WisniewskaNo ratings yet
- BrainDocument3 pagesBrainWidiya BudiNo ratings yet
- Circulatory SystemDocument10 pagesCirculatory SystemKent Clark VillaNo ratings yet
- Fundoscopy Made Easy Ming 2009Document34 pagesFundoscopy Made Easy Ming 2009Laura100% (1)
- Module 6Document33 pagesModule 6Sire Rose GepigaNo ratings yet
- Diagnosing Thoracic Outlet SyndromeDocument14 pagesDiagnosing Thoracic Outlet SyndromeolaNo ratings yet
- Honey Lorie D. Simbajon - Brain Injury Part IDocument7 pagesHoney Lorie D. Simbajon - Brain Injury Part IMary Grace SimbajonNo ratings yet
- Interstitial Lung DiseaseDocument14 pagesInterstitial Lung DiseaseAzkaZulfiqarNo ratings yet
- Part 2 Anatomy and PhysiologyDocument8 pagesPart 2 Anatomy and Physiologyzy- SBGNo ratings yet
- Multiple Choice Questions: A. B. C. DDocument55 pagesMultiple Choice Questions: A. B. C. DwanderagroNo ratings yet
- September 18 Animal Anatomy and PhysiologyDocument16 pagesSeptember 18 Animal Anatomy and Physiologyapi-525925794No ratings yet
- DivergentesDocument12 pagesDivergentesA LunaNo ratings yet
- Early Behavioral Intervention, Brain Plasticity, and The Prevention of Autism Spectrum DisorderDocument29 pagesEarly Behavioral Intervention, Brain Plasticity, and The Prevention of Autism Spectrum DisorderНиколија АндрићNo ratings yet