Professional Documents
Culture Documents
5 Lewis Acid and Base S201
5 Lewis Acid and Base S201
Uploaded by
AatishImroz0 ratings0% found this document useful (0 votes)
77 views1 pageThis document contains a chemistry problem involving identifying Lewis acids and bases in 4 reactions. In reaction a, ethanol is the Lewis base that donates an electron pair to the Lewis acid carbonic acid. In reaction b, the bromoalkane is the Lewis acid that accepts an electron pair from the methoxide ion Lewis base. Reaction c shows the chloroalkane as the Lewis acid accepting an electron pair from the amide ion Lewis base. And in reaction d, boron trifluoride acts as the Lewis acid accepting an electron pair from the chlorate ion Lewis base.
Original Description:
Original Title
5-lewis-acid-and-base-S201.doc
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document contains a chemistry problem involving identifying Lewis acids and bases in 4 reactions. In reaction a, ethanol is the Lewis base that donates an electron pair to the Lewis acid carbonic acid. In reaction b, the bromoalkane is the Lewis acid that accepts an electron pair from the methoxide ion Lewis base. Reaction c shows the chloroalkane as the Lewis acid accepting an electron pair from the amide ion Lewis base. And in reaction d, boron trifluoride acts as the Lewis acid accepting an electron pair from the chlorate ion Lewis base.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
77 views1 page5 Lewis Acid and Base S201
5 Lewis Acid and Base S201
Uploaded by
AatishImrozThis document contains a chemistry problem involving identifying Lewis acids and bases in 4 reactions. In reaction a, ethanol is the Lewis base that donates an electron pair to the Lewis acid carbonic acid. In reaction b, the bromoalkane is the Lewis acid that accepts an electron pair from the methoxide ion Lewis base. Reaction c shows the chloroalkane as the Lewis acid accepting an electron pair from the amide ion Lewis base. And in reaction d, boron trifluoride acts as the Lewis acid accepting an electron pair from the chlorate ion Lewis base.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 1
Student Name:
#5 Lewis Acid and Lewis Base
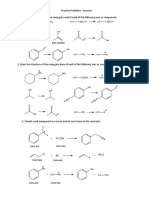
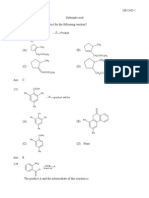
1. For each of the following reactions, draw the Lewis dot structure for each
reactant and product. Identify each reactant as a Lewis acid or a Lewis base.
a. CH3CH2CH2CH2CH2OH + CF3COOH CH3CH2CH2CH2CH2OH2+ + CF3COO-
b. CH3CH2CH2CH2Br + CH3O- CH3CH2CH2CH2OCH3 + Br-
(the O is between the last 2 C)
c. CH3CHClCH2CH3 + NH2- CH3CHCHCH3 + NH3 + Cl-
(for this problem only consider the new bond between N and H)
d. BF3 + OCl- BF3OCl
You might also like
- Balancing Redox Equations Q & ADocument40 pagesBalancing Redox Equations Q & AEw AldoNo ratings yet
- Redox Practice ProblemsDocument3 pagesRedox Practice ProblemsPeter Greener100% (1)
- Chapter 5 HydrocarbonDocument25 pagesChapter 5 Hydrocarbonmeshal retteryNo ratings yet
- Bansal Classes Organic Chemistry Study Material For IIT JEEDocument477 pagesBansal Classes Organic Chemistry Study Material For IIT JEEAditya Kavuluri40% (5)
- Chapter 24 ProblemsDocument13 pagesChapter 24 Problemslynette-wuNo ratings yet
- F AlkanesAlkenesStereochemTutorial 3Document4 pagesF AlkanesAlkenesStereochemTutorial 3Leong Yue YanNo ratings yet
- Chapter 2 ExrecicesDocument24 pagesChapter 2 Exrecicespaulinhagraebin100% (4)
- Worksheet 5 (Acids-Bases III) With AnswersDocument2 pagesWorksheet 5 (Acids-Bases III) With AnswersDelilah StephenieNo ratings yet
- CHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsDocument15 pagesCHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsSara Yuen100% (1)
- Oganic Cem PDFDocument2 pagesOganic Cem PDFaulaNo ratings yet
- Assignment 2 CHEM 215Document6 pagesAssignment 2 CHEM 215Abdullah AlteneijiNo ratings yet
- CY2102Document3 pagesCY2102Prarabdha SharmaNo ratings yet
- UAB Wang Recitation #1Document4 pagesUAB Wang Recitation #1OmarBilbeisiNo ratings yet
- Tutorial Alkyne 1Document6 pagesTutorial Alkyne 1aeleef patrick A20ET0356No ratings yet
- Chemistry Ii (Ecf 0024) Tutorial 5: CH CHCH CHCH OHDocument3 pagesChemistry Ii (Ecf 0024) Tutorial 5: CH CHCH CHCH OHutpNo ratings yet
- T12 Introduction To Organic Chemistry 27-34Document8 pagesT12 Introduction To Organic Chemistry 27-34饶宝珍No ratings yet
- Balancing Chemical EquationDocument15 pagesBalancing Chemical EquationPatricia Cadacio100% (1)
- Hcno C H H H C H H H C H H H: Remains +1 ThroughoutDocument4 pagesHcno C H H H C H H H C H H H: Remains +1 ThroughoutpNo ratings yet
- General Organic Chemistry-02 - Solved ProblemsDocument10 pagesGeneral Organic Chemistry-02 - Solved ProblemsRaju SinghNo ratings yet
- CH CH CCH C CHDocument15 pagesCH CH CCH C CHVirgilio Ebajo Jr.No ratings yet
- Goc - D23 Jan 2024Document25 pagesGoc - D23 Jan 2024hetansh2404No ratings yet
- Organic Chemistry: Preparations of Carbonyl Compounds Subjective QuestionsDocument6 pagesOrganic Chemistry: Preparations of Carbonyl Compounds Subjective QuestionsVandana ReddyNo ratings yet
- New Light Classes: 12B-Che Date-14/01/2022Document3 pagesNew Light Classes: 12B-Che Date-14/01/2022ShashwatNo ratings yet
- CHEM 203 Midterm Exam 2Document7 pagesCHEM 203 Midterm Exam 2pNo ratings yet
- HW 1 KeyDocument6 pagesHW 1 Keywinadoo87789697No ratings yet
- Problems: NomenclatureDocument7 pagesProblems: NomenclatureDũng TrầnNo ratings yet
- Carbonyl Compounds 1Document23 pagesCarbonyl Compounds 1Gowri ShankarNo ratings yet
- CY1101 Mid SemDocument3 pagesCY1101 Mid SemDipti Ranjan SahooNo ratings yet
- FINAL EXAMINATION in Analytical Chemistry Name: - Yr & Sec: - DateDocument3 pagesFINAL EXAMINATION in Analytical Chemistry Name: - Yr & Sec: - DateDivine Grace ValenzuelaNo ratings yet
- Orgo 2 GuideDocument25 pagesOrgo 2 GuideRukiezillaNo ratings yet
- Problem Set 2Document8 pagesProblem Set 2Katrina Louise GonzalesNo ratings yet
- Exercises General Chemistry II: H + CR O + C H OH (L) CR + Co (G) + H O (L)Document4 pagesExercises General Chemistry II: H + CR O + C H OH (L) CR + Co (G) + H O (L)Lê Anh VũNo ratings yet
- XI Mid Term QPDocument3 pagesXI Mid Term QPtechnical SiteNo ratings yet
- Chem Pu IDocument2 pagesChem Pu IMahesh HugarNo ratings yet
- Anic Chemistry Carboxylic AcidsDocument3 pagesAnic Chemistry Carboxylic AcidseamcetmaterialsNo ratings yet
- CHM207 TutorialDocument3 pagesCHM207 Tutorialit's miaNo ratings yet
- CBSE Class 12 Chemistry 2018Document17 pagesCBSE Class 12 Chemistry 2018parv dhanoteNo ratings yet
- Quiz 1 Sko3033 PDFDocument4 pagesQuiz 1 Sko3033 PDFFiona Tiwon100% (1)
- Latmer ExerciciosDocument7 pagesLatmer ExerciciosLilian WeitzelNo ratings yet
- ChemDocument6 pagesChembighneshrath1No ratings yet
- CadDocument8 pagesCadRamesh Babu GarlapatiNo ratings yet
- Organic Chemistry 3A Additional Problems Final Exam Part 1Document7 pagesOrganic Chemistry 3A Additional Problems Final Exam Part 1John SmithNo ratings yet
- Topic 10 Alkane TutorialDocument6 pagesTopic 10 Alkane TutorialTimNo ratings yet
- Chemistry, BT-2, SET-IDocument6 pagesChemistry, BT-2, SET-ISoham NagNo ratings yet
- CU-2020 B.Sc. (Honours) Chemistry Semester-III Paper-CC-7 QPDocument4 pagesCU-2020 B.Sc. (Honours) Chemistry Semester-III Paper-CC-7 QPbuntyckbtNo ratings yet
- Topic 1 - Carbon Compounds & Chemical Bonds: Tutorial: CHM125 - Basic Organic ChemistryDocument2 pagesTopic 1 - Carbon Compounds & Chemical Bonds: Tutorial: CHM125 - Basic Organic ChemistryFarhana Mohd RazaliNo ratings yet
- Elemental BalanceDocument15 pagesElemental BalanceAlanChevalNo ratings yet
- Os 7 Ha GCut ZG68 Gao OMk 1Document22 pagesOs 7 Ha GCut ZG68 Gao OMk 1Moist CottonCandyNo ratings yet
- Worksheet For Organic SectionDocument17 pagesWorksheet For Organic SectionPramudith Liyanage100% (2)
- 2423 e 2Document24 pages2423 e 2Agustin KurniatiNo ratings yet
- Name of The Molecule Molecular Formula Hybridization Type Bond Angle GeometryDocument7 pagesName of The Molecule Molecular Formula Hybridization Type Bond Angle GeometryKerala MekuriyaNo ratings yet
- ENVE102 Recitation5 SolutionsDocument5 pagesENVE102 Recitation5 Solutionsdtokat04No ratings yet
- 16 Introduction To Organic ChemistryDocument9 pages16 Introduction To Organic Chemistryizabel0% (1)
- General Chemistry I: Final Exams Review PacketDocument20 pagesGeneral Chemistry I: Final Exams Review PacketEdmark LuspeNo ratings yet
- Test1 342 PracticeV1Document5 pagesTest1 342 PracticeV1Camha NguyenNo ratings yet
- Revision A Level 2022 QPDocument3 pagesRevision A Level 2022 QPJulianNo ratings yet
- Problems For Chapter 1 & 2 ANSWERS: 2xH 2 2xN 10 O 6Document6 pagesProblems For Chapter 1 & 2 ANSWERS: 2xH 2 2xN 10 O 6JibrilAttawarahNo ratings yet
- It May Happen That Such A Filter Is To Be Used In: Involves Using A Multiple-Cavity Fabry-Perot Filter To Achieve The NBPDocument13 pagesIt May Happen That Such A Filter Is To Be Used In: Involves Using A Multiple-Cavity Fabry-Perot Filter To Achieve The NBPAatishImrozNo ratings yet
- Towards Longwave Infrared Tuneable Filters For Multispectral Thermal Imaging ApplicationsDocument6 pagesTowards Longwave Infrared Tuneable Filters For Multispectral Thermal Imaging ApplicationsAatishImrozNo ratings yet
- Anisotropic Magnetoresistance Ferromagnetic Alloys: Ap Ap/pavr PavDocument21 pagesAnisotropic Magnetoresistance Ferromagnetic Alloys: Ap Ap/pavr PavAatishImrozNo ratings yet
- Longitudinal and Transverse Magnetoresistance in Films With Tilted Out-Of-Plane Magnetic AnisotropyDocument34 pagesLongitudinal and Transverse Magnetoresistance in Films With Tilted Out-Of-Plane Magnetic AnisotropyAatishImrozNo ratings yet
- E2T2 PracticeDocument12 pagesE2T2 PracticeAatishImrozNo ratings yet
- MULTIPLE CHOICE. Choose The One Alternative That Best Completes The Statement or Answers The QuestionDocument5 pagesMULTIPLE CHOICE. Choose The One Alternative That Best Completes The Statement or Answers The QuestionAatishImrozNo ratings yet
- Study Guide Unit 2Document8 pagesStudy Guide Unit 2AatishImrozNo ratings yet
- 11 Integrated Rate Law S201 1Document5 pages11 Integrated Rate Law S201 1AatishImroz0% (1)
- 14 Rate Constant and Temp S202 1Document1 page14 Rate Constant and Temp S202 1AatishImrozNo ratings yet
- 12 Potential Energy Diagram S201 1Document2 pages12 Potential Energy Diagram S201 1AatishImrozNo ratings yet
- 10 Determining Rate Law Expression From Data S201 1Document4 pages10 Determining Rate Law Expression From Data S201 1AatishImrozNo ratings yet
- ch27 PDFDocument87 pagesch27 PDFAatishImrozNo ratings yet