Professional Documents
Culture Documents
Quiz C6 Set 1
Uploaded by
Supia Nazma0 ratings0% found this document useful (0 votes)
10 views2 pagesThis document contains a 3 question quiz on chemical equilibrium for students at Kolej Matrikulasi Kedah. The quiz asks students to:
1) Draw the concentration versus time graph for a reversible reaction of A + B ⇌ C + D.
2) Write the expressions for Kc and Kp for the reaction NO2(g) ⇌ NO(g) + 1/2O2(g) and describe two differences between Kc and Kp.
3) Calculate the value of Kp given that for the reaction CO(g) + H2O(g) ⇌ CO2(g) + H2(g), Kc = 4.
Original Description:
Original Title
QUIZ C6 SET 1
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document contains a 3 question quiz on chemical equilibrium for students at Kolej Matrikulasi Kedah. The quiz asks students to:
1) Draw the concentration versus time graph for a reversible reaction of A + B ⇌ C + D.
2) Write the expressions for Kc and Kp for the reaction NO2(g) ⇌ NO(g) + 1/2O2(g) and describe two differences between Kc and Kp.
3) Calculate the value of Kp given that for the reaction CO(g) + H2O(g) ⇌ CO2(g) + H2(g), Kc = 4.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views2 pagesQuiz C6 Set 1
Uploaded by
Supia NazmaThis document contains a 3 question quiz on chemical equilibrium for students at Kolej Matrikulasi Kedah. The quiz asks students to:
1) Draw the concentration versus time graph for a reversible reaction of A + B ⇌ C + D.
2) Write the expressions for Kc and Kp for the reaction NO2(g) ⇌ NO(g) + 1/2O2(g) and describe two differences between Kc and Kp.
3) Calculate the value of Kp given that for the reaction CO(g) + H2O(g) ⇌ CO2(g) + H2(g), Kc = 4.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 2
DK025
Semester II 2013/2014
SET 1
KOLEJ MATRIKULASI KEDAH
QUIZ (CHEMICAL EQUILIBRIUM )
______________________________________________________________________ _______
NAME:__________________________________ TUTORIAL:_________
Answer all the question.
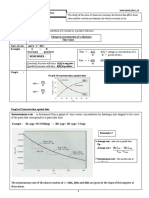
1. Draw the graph for concentration versus time for reversible reaction?
Chemical reaction is given below:
A + B C + D
[ 2 marks ]
2. Write Kc and Kp for the reaction:
NO2 (g) NO (g) + ½ O2 (g)
Give two different between Kc and Kp?
[ 4 marks ]
3. Given
CO (g) + H2O (g) CO2 (g) + H2 (g)
At 500 oC, Kc = 4.05 at this temperature. [R = 0.0821 L atm mol-1 K-1]
Determine Kp for the reaction.
[ 4 marks ]
DK025
Semester II 2013/2014
SET 1
You might also like
- Quiz C6 Set 3Document1 pageQuiz C6 Set 3Supia NazmaNo ratings yet
- Quiz C6 Set 2Document2 pagesQuiz C6 Set 2Supia NazmaNo ratings yet
- Kolej Matrikulasi KedahDocument2 pagesKolej Matrikulasi KedahkjjkimkmkNo ratings yet
- 6.2 Chemical Equilbrium-Pelajar 20 JulaiDocument58 pages6.2 Chemical Equilbrium-Pelajar 20 JulaiAisyah AzahariNo ratings yet
- Chemistry Sk025 SESSION 2019/2020 Topic: ThermochemistryDocument3 pagesChemistry Sk025 SESSION 2019/2020 Topic: ThermochemistryHaiyi GohNo ratings yet
- Apch16 ps1 06Document2 pagesApch16 ps1 06aoiwefoweiNo ratings yet
- Exercise - V: JEE-ProblemsDocument1 pageExercise - V: JEE-ProblemsAmudala HemashviniNo ratings yet
- Tutorial Sheet4Document4 pagesTutorial Sheet4Lê Anh QuangNo ratings yet
- Y13 PPE 2022 Paper 1 CompleteDocument14 pagesY13 PPE 2022 Paper 1 CompleteDehabNo ratings yet
- AssociationDocument2 pagesAssociationRichmond EresmasNo ratings yet
- Spek DronDocument12 pagesSpek DronChem MistryNo ratings yet
- Applicationsof HessslawDocument92 pagesApplicationsof Hessslaw/ “Nu” /No ratings yet
- Equilibrium DPP 014728Document11 pagesEquilibrium DPP 014728Yash MalviyaNo ratings yet
- Question Paper Periodic Table Elements and Physical ChemistryDocument28 pagesQuestion Paper Periodic Table Elements and Physical ChemistryEsam ELNOAMANYNo ratings yet
- Module 13-14 NotesDocument12 pagesModule 13-14 Notesjared.greenwood93No ratings yet
- Enthalpy WKSTDocument3 pagesEnthalpy WKSTfernandezshanaya35No ratings yet
- Energetics Exam Q BookletDocument16 pagesEnergetics Exam Q BookletEmoryNo ratings yet
- Exam Style Answers 6Document3 pagesExam Style Answers 6Thanos GamingNo ratings yet
- A2 Chemistry Gibbs Free Energy Change WSDocument2 pagesA2 Chemistry Gibbs Free Energy Change WSnoreenaz575No ratings yet
- Chemical Equilibrium Post LabDocument53 pagesChemical Equilibrium Post LabJimilyn Michelle HofeleñaNo ratings yet
- 7 Equilibrium: SolutionsDocument54 pages7 Equilibrium: SolutionsMriganko RoyNo ratings yet
- Phy Chem ProblemsDocument5 pagesPhy Chem ProblemsPatricia de LeonNo ratings yet
- ChemistryDocument23 pagesChemistryDũng HoàngNo ratings yet
- Complete PPT On Chemical EquilibriaDocument52 pagesComplete PPT On Chemical EquilibriaSaikiran Chamakuri71% (7)
- AP 2006 Chemistry - Scoring GuidelinesDocument16 pagesAP 2006 Chemistry - Scoring GuidelinesDiane LeeNo ratings yet
- HL SummDocument12 pagesHL SummWilliam AungkhantNo ratings yet
- Cambridge International AS & A Level: CHEMISTRY 9701/51Document12 pagesCambridge International AS & A Level: CHEMISTRY 9701/51Slamet HarionoNo ratings yet
- Chapter 13 - Chemical EquilibriumDocument52 pagesChapter 13 - Chemical EquilibriummukhlishNo ratings yet
- Answers: Rate K (H) (NO) Must Determine A and BDocument4 pagesAnswers: Rate K (H) (NO) Must Determine A and BWahyu YusupNo ratings yet
- Ana Chem For Engineers Q1 2020 PDFDocument2 pagesAna Chem For Engineers Q1 2020 PDFSakamaki IzayoiNo ratings yet
- J1 Promos 2015 Paper 1Document11 pagesJ1 Promos 2015 Paper 1aliciaNo ratings yet
- Chemistry KS4 LZ 2.2Document16 pagesChemistry KS4 LZ 2.2hiiamoskalawΛwΛ /-No ratings yet
- Hess - S Law Pack 1Document13 pagesHess - S Law Pack 1bilaalquadriNo ratings yet
- Entropy TestDocument9 pagesEntropy TestSahanNivanthaNo ratings yet
- Problem Set #26 - Chem 141 PracticeDocument1 pageProblem Set #26 - Chem 141 PracticejennygrahamsmithNo ratings yet
- CLASS 12 PHYSICAL Diwali Assignment ChemistryDocument8 pagesCLASS 12 PHYSICAL Diwali Assignment ChemistryPrashantNo ratings yet
- G = Δh - Tδs: Spontaneity and Gibbs free energyDocument4 pagesG = Δh - Tδs: Spontaneity and Gibbs free energyAdufe RufaiNo ratings yet
- 132 E4 Review S15Document16 pages132 E4 Review S15DHNo ratings yet
- Modifying Equilibrium Constant Expressions:: (G) (G) (G) (G) (G) (G) (G) (G) (G) (G)Document1 pageModifying Equilibrium Constant Expressions:: (G) (G) (G) (G) (G) (G) (G) (G) (G) (G)Raffy FlandesNo ratings yet
- 5.2 (152 Marks) : 1. (1 Mark)Document42 pages5.2 (152 Marks) : 1. (1 Mark)Semwezi EnockNo ratings yet
- Award (1) Each For Any Two. Accept Energy Instead of Heat.: IB Questionbank Chemistry 1Document4 pagesAward (1) Each For Any Two. Accept Energy Instead of Heat.: IB Questionbank Chemistry 1Jen JenNo ratings yet
- Energetics SL - MCQDocument22 pagesEnergetics SL - MCQAster LeeNo ratings yet
- Year 12 Enthalpy Changes February Half TermDocument28 pagesYear 12 Enthalpy Changes February Half TermEri-ife OlufemiNo ratings yet
- CHE 107 Exam 2 Spring 2016: Your Name: Your ID: Question #Document20 pagesCHE 107 Exam 2 Spring 2016: Your Name: Your ID: Question #NavneetNo ratings yet
- Theme: Matter in Nature: Learning Area: 1. Land and Its ResourcesDocument17 pagesTheme: Matter in Nature: Learning Area: 1. Land and Its ResourcesChee Jin TangNo ratings yet
- Question Paper Periodic Table Elements and Physical ChemistryDocument32 pagesQuestion Paper Periodic Table Elements and Physical ChemistryChi Wang LAWNo ratings yet
- Higher Technological Institute Chemical Engineering DepartmentDocument10 pagesHigher Technological Institute Chemical Engineering Departmentlove youNo ratings yet
- Activity 2 Chemical EquilibriumDocument1 pageActivity 2 Chemical EquilibriumAira DeomanoNo ratings yet
- 2 Quizizz 2019 ptVIIIe DocDocument10 pages2 Quizizz 2019 ptVIIIe DocKM Tsang Ka ManNo ratings yet
- Quiz C6 Set 4Document2 pagesQuiz C6 Set 4Supia NazmaNo ratings yet
- Equillibrium Worksheet 2Document15 pagesEquillibrium Worksheet 2Rahayu CamscanNo ratings yet
- Chemical EquilibriumDocument2 pagesChemical EquilibriumShivani VermaNo ratings yet
- DPP-5 - Student Copy (Chemical Equlibrium)Document4 pagesDPP-5 - Student Copy (Chemical Equlibrium)prashantyadavpky07No ratings yet
- Thermochemistry PC EDocument12 pagesThermochemistry PC Eb72hbapqiNo ratings yet
- 27 MARCH 2020: Assignment 5 Question PaperDocument4 pages27 MARCH 2020: Assignment 5 Question PaperShadreck SandweNo ratings yet
- Name - Honors Chemistry - / - / - Hess's LawDocument4 pagesName - Honors Chemistry - / - / - Hess's LawGunjee GunjeeNo ratings yet
- ChemistryDocument24 pagesChemistryZae ValentineNo ratings yet
- (CO5) Chemical EquilibriumDocument35 pages(CO5) Chemical EquilibriumAya Evangelista AlmandresNo ratings yet
- Chem T7 HLQDocument6 pagesChem T7 HLQSarah KohNo ratings yet
- Model Answers in Ordinary National Certificate Mathematics for EngineersFrom EverandModel Answers in Ordinary National Certificate Mathematics for EngineersNo ratings yet
- Chapter 1: Matter 1.1 Atoms and Molecules: Packed in A Small NucleusDocument35 pagesChapter 1: Matter 1.1 Atoms and Molecules: Packed in A Small NucleusSupia NazmaNo ratings yet
- 2 Metallic BondsDocument13 pages2 Metallic BondsSupia NazmaNo ratings yet
- Quiz PHASE EQUILIBRIA (Set 3)Document4 pagesQuiz PHASE EQUILIBRIA (Set 3)Supia NazmaNo ratings yet
- Instantaneous Rate: Is Determined From A Graph of Time Versus Concentration by Drawing A Line Tangent To The CurveDocument13 pagesInstantaneous Rate: Is Determined From A Graph of Time Versus Concentration by Drawing A Line Tangent To The CurveSupia NazmaNo ratings yet
- Exercise Born HaberDocument17 pagesExercise Born HaberSupia NazmaNo ratings yet
- Exercise Born HaberDocument17 pagesExercise Born HaberSupia NazmaNo ratings yet
- Quiz PHASE EQUILIBRIA (Set 2)Document4 pagesQuiz PHASE EQUILIBRIA (Set 2)Supia NazmaNo ratings yet
- Extra Exercises - Measurement of ConcentrationDocument1 pageExtra Exercises - Measurement of ConcentrationSupia NazmaNo ratings yet
- Collision Theory States That For A Reaction To OccurDocument9 pagesCollision Theory States That For A Reaction To OccurSupia NazmaNo ratings yet
- Quiz 1.1 2021 LectureDocument4 pagesQuiz 1.1 2021 LectureSupia NazmaNo ratings yet
- Quiz C5 STATES OF MATTER (Set 5)Document2 pagesQuiz C5 STATES OF MATTER (Set 5)Supia NazmaNo ratings yet
- Quiz C6 Set 4Document2 pagesQuiz C6 Set 4Supia NazmaNo ratings yet
- Topic 14.0: Haloalkanes (Alkyl Halides)Document12 pagesTopic 14.0: Haloalkanes (Alkyl Halides)Supia NazmaNo ratings yet
- Gases (B)Document115 pagesGases (B)Supia NazmaNo ratings yet
- Quiz States of Matter (Set 4)Document4 pagesQuiz States of Matter (Set 4)Supia NazmaNo ratings yet
- 7.0 Ionic Equilibria (Students)Document187 pages7.0 Ionic Equilibria (Students)Supia Nazma100% (1)
- CARBOXYLIC ACIDS Nomenclature StudentDocument23 pagesCARBOXYLIC ACIDS Nomenclature StudentSupia NazmaNo ratings yet
- Tutorial 1.1 (PG 1-2)Document3 pagesTutorial 1.1 (PG 1-2)Supia NazmaNo ratings yet
- Topic 14.0: Haloalkanes (Alkyl Halides)Document12 pagesTopic 14.0: Haloalkanes (Alkyl Halides)Supia NazmaNo ratings yet
- Gaya Komunikasi Ketua Unit (Ku) Kimia Dan Kepuasan Kerja Pensyarah Kimia Di Kolej Matrikulasi SelangorDocument12 pagesGaya Komunikasi Ketua Unit (Ku) Kimia Dan Kepuasan Kerja Pensyarah Kimia Di Kolej Matrikulasi SelangorSupia NazmaNo ratings yet
- Set 1 Lampiran 1C - PelajarDocument1 pageSet 1 Lampiran 1C - PelajarSupia NazmaNo ratings yet
- Worksheet 1Document6 pagesWorksheet 1Supia NazmaNo ratings yet
- 19 (B)Document4 pages19 (B)Supia NazmaNo ratings yet
- CHC NH Cooh H H CH C CH: Organic Compound That Contains Both An Amino Group, - NH2 and A Carboxyl Group, - COOHDocument6 pagesCHC NH Cooh H H CH C CH: Organic Compound That Contains Both An Amino Group, - NH2 and A Carboxyl Group, - COOHSupia NazmaNo ratings yet
- Chapter 4.4-Intermolecular ForcesDocument3 pagesChapter 4.4-Intermolecular ForcesSupia NazmaNo ratings yet