Professional Documents
Culture Documents
K. Polymerization: Table IV: 2-16. Polymerization Process
Uploaded by
Phi TiêuOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
K. Polymerization: Table IV: 2-16. Polymerization Process
Uploaded by
Phi TiêuCopyright:
Available Formats
K.
Polymerization
Polymerization in the petroleum industry is the process of converting light olefin gases
including ethylene, propylene, and butylene into hydrocarbons of higher molecular weight
and higher octane number that can be used as gasoline blending stocks. Polymerization
combines two or more identical olefin molecules to form a single molecule with the same
elements in the same proportions as the original molecules. Polymerization may be
accomplished thermally or in the presence of a catalyst at lower temperatures.
The olefin feedstock is pretreated to remove sulfur and other undesirable compounds. In the
catalytic process the feedstock is either passed over a solid phosphoric acid catalyst or comes
in contact with liquid phosphoric acid, where an exothermic polymeric reaction occurs. This
reaction requires cooling water and the injection of cold feedstock into the reactor to control
temperatures between 300º and 450º F at pressures from 200 psi to 1,200 psi. The reaction
products leaving the reactor are sent to stabilization and/or fractionator systems to separate
saturated and unreacted gases from the polymer gasoline product.
NOTE: In the petroleum industry, polymerization is used to indicate the production of
gasoline components, hence the term "polymer" gasoline. Furthermore, it is not essential that
only one type of monomer be involved. If unlike olefin molecules are combined, the process
is referred to as "copolymerization." Polymerization in the true sense of the word is normally
prevented, and all attempts are made to terminate the reaction at the dimer or trimer (three
monomers joined together) stage. However, in the petrochemical section of a refinery,
polymerization, which results in the production of, for instance, polyethylene, is allowed to
proceed until materials of the required high molecular weight have been produced.
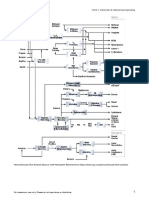
Table IV: 2-16. Polymerization Process
Feedstock From Process Typical products . . . . . . To
Olefins Cracking Unification High octane naphtha . . . . Gasoline blending
processes Petrochem. feedstock . . . . Petrochemical
Liquid petro. gas . . . . . . . Storage
Figure IV: 2-20. Polymerization Process
SAFETY AND HEALTH CONSIDERATIONS
Fire Prevention and Protection
Polymerization is a closed process where the potential for a fire could occur due to leaks or
releases reaching a source of ignition.
Safety
The potential for an uncontrolled exothermic reaction exists should loss of cooling water
occur. Severe corrosion leading to equipment failure will occur should water make contact
with the phosphoric acid, such as during water washing at shutdowns. Corrosion may also
occur in piping manifolds, reboilers, exchangers, and other locations where acid may settle
out.
Health
Because this is a closed system, exposures are expected to be minimal under normal
operating conditions. There is a potential for exposure to caustic wash (sodium hydroxide), to
phosphoric
acid used in the process or washed out during turnarounds, and to catalyst dust. Safe work
practices and/or appropriate personal protective equipment may be needed for exposures to
chemicals and other hazards such as noise and heat, and during process sampling, inspection,
maintenance, and turnaround activities.
You might also like
- Nutrition & You - Chapter 6Document40 pagesNutrition & You - Chapter 6Bridget KathleenNo ratings yet
- Petroleum Technology-Part Iii: The Process & Technology of CrackingDocument23 pagesPetroleum Technology-Part Iii: The Process & Technology of CrackingSrikrishnan KrishNo ratings yet
- Voith-Paper Twogether24 enDocument76 pagesVoith-Paper Twogether24 enPhi TiêuNo ratings yet
- Polyether Polyol Production AssignmentDocument9 pagesPolyether Polyol Production AssignmentanurdiaNo ratings yet
- Transfer and Business Taxation SyllabusDocument5 pagesTransfer and Business Taxation SyllabusamqqndeahdgeNo ratings yet
- Business Plan Example - Little LearnerDocument26 pagesBusiness Plan Example - Little LearnerCourtney mcintosh100% (1)
- Assignment 2 Sample PDFDocument78 pagesAssignment 2 Sample PDFNg SiewminNo ratings yet
- Heavy and Extra-heavy Oil Upgrading TechnologiesFrom EverandHeavy and Extra-heavy Oil Upgrading TechnologiesRating: 4 out of 5 stars4/5 (2)
- Chemical Recycling of PETDocument39 pagesChemical Recycling of PETAmit SinghNo ratings yet
- Neopuff PDFDocument4 pagesNeopuff PDFoechimNo ratings yet
- Hydrocarbon ProcessingDocument20 pagesHydrocarbon Processingsanjeevs01No ratings yet
- Assignment NO 3:: Question 1: Define Cracking. Classify Cracking OperationsDocument6 pagesAssignment NO 3:: Question 1: Define Cracking. Classify Cracking OperationsMilan MoradiyaNo ratings yet
- Itc LimitedDocument64 pagesItc Limitedjulee G0% (1)
- Lesson 1 - Intro To Highway EngineeringDocument15 pagesLesson 1 - Intro To Highway EngineeringSaoirseNo ratings yet
- University of Engineering & Technology KSK New Campus: BiomassDocument11 pagesUniversity of Engineering & Technology KSK New Campus: BiomassAli Raza100% (1)
- Modelling of Naphtha Cracking For Olefins Production - Joao MarcosDocument9 pagesModelling of Naphtha Cracking For Olefins Production - Joao MarcosBahar MeschiNo ratings yet
- Lecture2 (Petrochemical)Document12 pagesLecture2 (Petrochemical)ToniAndiwijaya100% (1)
- Direct Methane to Methanol: Foundations and Prospects of the ProcessFrom EverandDirect Methane to Methanol: Foundations and Prospects of the ProcessNo ratings yet
- GTC Isomalk Technologies For Light Naphtha IsomerizationDocument29 pagesGTC Isomalk Technologies For Light Naphtha IsomerizationToni ĐạtNo ratings yet
- Pyrolysis Furnace Rev 1 PDFDocument11 pagesPyrolysis Furnace Rev 1 PDFKmajdianNo ratings yet
- Steam Cracking Kinetics and Feed Characterisation PDFDocument10 pagesSteam Cracking Kinetics and Feed Characterisation PDFBehroozNo ratings yet
- 7 - OligomerizationDocument16 pages7 - OligomerizationAn Lê TrườngNo ratings yet
- 3 Coking ProcessesDocument26 pages3 Coking ProcessesFranklin RevillNo ratings yet
- Petroleum Technology Two MarkDocument26 pagesPetroleum Technology Two Markdhanagopal saiNo ratings yet
- Bedwetting TCMDocument5 pagesBedwetting TCMRichonyouNo ratings yet
- PetrochemicalsDocument72 pagesPetrochemicalsSaiPavanNo ratings yet
- Cracking 151114162534 Lva1 App6891Document33 pagesCracking 151114162534 Lva1 App6891Truth SeekerNo ratings yet
- PRP Unit - 6Document4 pagesPRP Unit - 6Abdul GhafoorNo ratings yet
- Assignment 1Document8 pagesAssignment 1Sulman KhalidNo ratings yet
- Assignment 1Document7 pagesAssignment 1Pratiksha GoreNo ratings yet
- Ass 1Document6 pagesAss 1Pratiksha GoreNo ratings yet
- Group WorkDocument4 pagesGroup WorkRuva Oscass JimmyNo ratings yet
- Tas Institute of OilDocument7 pagesTas Institute of OilSheena DovenantNo ratings yet
- XV Paper 19Document13 pagesXV Paper 19Raghav SharmaNo ratings yet
- Unit-V 13Document16 pagesUnit-V 13Kooki KumarNo ratings yet
- Chapter 7Document18 pagesChapter 7Ameer AlawadiNo ratings yet
- Chemical Technology (CHE1004) Module 5 Lecture 32: Secondary RefiningDocument28 pagesChemical Technology (CHE1004) Module 5 Lecture 32: Secondary RefiningSubham JhaNo ratings yet
- CBE697.TOPIC2.feedstock and Pretreatment2Document34 pagesCBE697.TOPIC2.feedstock and Pretreatment2SyahmiNo ratings yet
- Chapter 4Document26 pagesChapter 4indumathijayakaranNo ratings yet
- Feasibility StudyDocument15 pagesFeasibility StudytassoneNo ratings yet
- NHDT FoulingDocument6 pagesNHDT FoulingJulio RamirezNo ratings yet
- Plastic Industries: An OverviewDocument10 pagesPlastic Industries: An OverviewKubra ĖdrisNo ratings yet
- Lesage 23709Document8 pagesLesage 23709حاتم غيدان خلفNo ratings yet
- Lesage 23709Document8 pagesLesage 23709حاتم غيدان خلفNo ratings yet
- Ethylene Midterms PetrochemDocument8 pagesEthylene Midterms PetrochemEfraim AbuelNo ratings yet
- 01 Process ReactionDocument10 pages01 Process ReactionJacky WongNo ratings yet
- Catalytic Cracking3Document12 pagesCatalytic Cracking3Abdurabu AL-MontaserNo ratings yet
- Introduction Che 425Document2 pagesIntroduction Che 425فيصل الغامديNo ratings yet
- 2008-04 COLOHydroAromatic MustangDocument10 pages2008-04 COLOHydroAromatic MustangGarry DavidNo ratings yet
- Manufacturing of Dipheny CarbonateDocument5 pagesManufacturing of Dipheny CarbonatesadiaNo ratings yet
- Inf Ufc 85Document13 pagesInf Ufc 85Luciano Montellano Abasto100% (2)
- Introduction To Petrochemical ProcessesDocument23 pagesIntroduction To Petrochemical ProcessesHamed HadizadehNo ratings yet
- Report Control SystemDocument17 pagesReport Control SystemHussein Al HabebNo ratings yet
- Fixed-Bed Semi-Regenerative Platforming Process PDFDocument4 pagesFixed-Bed Semi-Regenerative Platforming Process PDFasifdcetNo ratings yet
- MethodsDocument4 pagesMethodsPolicNo ratings yet
- Degussa Developed Te First Industrial Acrolein Synthesis in The Early 1930sDocument6 pagesDegussa Developed Te First Industrial Acrolein Synthesis in The Early 1930sDasdsa SadadsaNo ratings yet
- Flowsheet MonsantoDocument3 pagesFlowsheet MonsantodelifaNo ratings yet
- Alkylation, Isomerisation and PolymerisationDocument9 pagesAlkylation, Isomerisation and Polymerisationapi-256504985No ratings yet
- Franz Fischer Hans Tropsch 1923 1925Document31 pagesFranz Fischer Hans Tropsch 1923 1925Jayakaran PachiyappanNo ratings yet
- Refinery BasicsDocument31 pagesRefinery Basicsanurag100% (1)
- 1 s2.0 S0926860X10001596 MainDocument8 pages1 s2.0 S0926860X10001596 Mainzahira.mohamedseghirNo ratings yet
- Petrochemical Technology: Boä Moân CNCB Daàu Khí, Khoa CNHH, ÑH Baùch Khoa Tp. HCMDocument25 pagesPetrochemical Technology: Boä Moân CNCB Daàu Khí, Khoa CNHH, ÑH Baùch Khoa Tp. HCMhatucdao2712No ratings yet
- 19.-Pyrolysis FurnaceDocument5 pages19.-Pyrolysis FurnaceWilfredo PastranaNo ratings yet
- Notes On Terminology and Technology in Thermal Conversion: DR Cordner Peacocke and DR Stephen JosephDocument5 pagesNotes On Terminology and Technology in Thermal Conversion: DR Cordner Peacocke and DR Stephen JosephArif Firman AjiNo ratings yet
- Poly (Vinyl Chloride) : 1.1 Raw MaterialDocument15 pagesPoly (Vinyl Chloride) : 1.1 Raw MaterialBhagat Singh RanaNo ratings yet
- Appendix 02. Lab Report Example 1Document5 pagesAppendix 02. Lab Report Example 1Linh LinhNo ratings yet
- Exxonmobil Fluid Bed MTG Overview enDocument13 pagesExxonmobil Fluid Bed MTG Overview enPhi TiêuNo ratings yet
- L1. An Interational IndustryDocument6 pagesL1. An Interational IndustryPhi TiêuNo ratings yet
- Exercise 1.2: Table 1. Distribution of Verb Tenses in Each Section of The Lab ReportDocument1 pageExercise 1.2: Table 1. Distribution of Verb Tenses in Each Section of The Lab ReportPhi TiêuNo ratings yet
- Appendix 02. Lab Report Example 1Document5 pagesAppendix 02. Lab Report Example 1Linh LinhNo ratings yet
- L1. An Interational IndustryDocument6 pagesL1. An Interational IndustryPhi TiêuNo ratings yet
- Exercise 1.2: Table 1. Distribution of Verb Tenses in Each Section of The Lab ReportDocument1 pageExercise 1.2: Table 1. Distribution of Verb Tenses in Each Section of The Lab ReportPhi TiêuNo ratings yet
- Advances in Catalysis For Methanol-to-Olefins ConversionDocument88 pagesAdvances in Catalysis For Methanol-to-Olefins ConversionPhi TiêuNo ratings yet
- Nadir Yilmaz, Francisco M. Vigil, Kyle Benalil, Stephen M. Davis, Antonio CalvaDocument5 pagesNadir Yilmaz, Francisco M. Vigil, Kyle Benalil, Stephen M. Davis, Antonio CalvaPhi TiêuNo ratings yet
- Biobutanol - Advantages and Disavantages. Comparision To Ethanol.Document9 pagesBiobutanol - Advantages and Disavantages. Comparision To Ethanol.Phi TiêuNo ratings yet
- ATMOS Technology: Premium Tissue With High Absorbency and BulkDocument6 pagesATMOS Technology: Premium Tissue With High Absorbency and BulkPhi TiêuNo ratings yet
- Fuel Cruel Oil Sectors Product Upstream DownstreamDocument6 pagesFuel Cruel Oil Sectors Product Upstream DownstreamPhi TiêuNo ratings yet
- 1 PB2Document38 pages1 PB2Phi TiêuNo ratings yet
- Bioresources.: Performance and Sustainability vs. The Shelf Price of Tissue Paper Kitchen TowelsDocument25 pagesBioresources.: Performance and Sustainability vs. The Shelf Price of Tissue Paper Kitchen TowelsPhi TiêuNo ratings yet
- Handbook Nitrogen Oxides Pollution Prevention and ControlDocument5 pagesHandbook Nitrogen Oxides Pollution Prevention and ControlrupigapigaNo ratings yet
- Global Inventory of NO Sources: R. Delmas, D. Ser Ca & C. JambertDocument10 pagesGlobal Inventory of NO Sources: R. Delmas, D. Ser Ca & C. JambertPhi TiêuNo ratings yet
- 4 CVieira CELPA CEF40years 0Document29 pages4 CVieira CELPA CEF40years 0Phi TiêuNo ratings yet
- United States Patent (19) : (73) Assignee: Scott Paper Company, DelawareDocument20 pagesUnited States Patent (19) : (73) Assignee: Scott Paper Company, DelawarePhi TiêuNo ratings yet
- How To Make A Simple Sponge Cake: Step 1: Preheat Oven To 350 Degrees FDocument1 pageHow To Make A Simple Sponge Cake: Step 1: Preheat Oven To 350 Degrees FPhi TiêuNo ratings yet
- Influence of Pressure & Catalytic ActivityDocument4 pagesInfluence of Pressure & Catalytic ActivityPhi TiêuNo ratings yet
- hóa học dầu mỏ và khíDocument5 pageshóa học dầu mỏ và khíPhi TiêuNo ratings yet
- American Ceramic: SocietyDocument8 pagesAmerican Ceramic: SocietyPhi TiêuNo ratings yet
- (MATH-EDUCARE) - Bai Tap Giai Tich Tran Duc Long - Tap 1 - Phan 2Document196 pages(MATH-EDUCARE) - Bai Tap Giai Tich Tran Duc Long - Tap 1 - Phan 2Phi TiêuNo ratings yet
- Hanoi University of Science & Technology Semester 20171 (WEB)Document1 pageHanoi University of Science & Technology Semester 20171 (WEB)Phi TiêuNo ratings yet
- Minyak Atsiri Sereh WangiDocument4 pagesMinyak Atsiri Sereh Wangicindy paraditha kasandraNo ratings yet
- VPD RashchrtDocument2 pagesVPD RashchrtMarco Ramos JacobNo ratings yet
- Gendec - Inbound HS-HTNDocument1 pageGendec - Inbound HS-HTNKhalidNo ratings yet
- Report On Analysis of TSF Water Samples Using Cyanide PhotometerDocument4 pagesReport On Analysis of TSF Water Samples Using Cyanide PhotometerEleazar DequiñaNo ratings yet
- 5SDD 71B0210Document4 pages5SDD 71B0210Merter TolunNo ratings yet
- Penilaian Akhir TahunDocument4 pagesPenilaian Akhir TahunRestu Suci UtamiNo ratings yet
- ReliabilityDocument5 pagesReliabilityArmajaya Fajar SuhardimanNo ratings yet
- Unit 8 Packet KeyDocument21 pagesUnit 8 Packet KeyHiddenNo ratings yet
- Ras Shastra PPT 6Document10 pagesRas Shastra PPT 6Soham BhureNo ratings yet
- FINAL PAPER Marketing Plan For Rainbow Air PurifierDocument12 pagesFINAL PAPER Marketing Plan For Rainbow Air PurifierMohola Tebello Griffith100% (1)
- Royal British College IncDocument5 pagesRoyal British College IncLester MojadoNo ratings yet
- READING 4 UNIT 8 Crime-Nurse Jorge MonarDocument3 pagesREADING 4 UNIT 8 Crime-Nurse Jorge MonarJORGE ALEXANDER MONAR BARRAGANNo ratings yet
- Join Our Telegram Channel: @AJITLULLA: To Get Daily Question Papers & SolutionsDocument24 pagesJoin Our Telegram Channel: @AJITLULLA: To Get Daily Question Papers & SolutionsNaveen KumarNo ratings yet
- Behavior Specific Praise Statements HandoutDocument3 pagesBehavior Specific Praise Statements HandoutDaniel BernalNo ratings yet
- Inducement of Rapid Analysis For Determination of Reactive Silica and Available Alumina in BauxiteDocument11 pagesInducement of Rapid Analysis For Determination of Reactive Silica and Available Alumina in BauxiteJAFAR MUHAMMADNo ratings yet
- ATI Respiratory PowerpointDocument90 pagesATI Respiratory PowerpointAnn KelseaNo ratings yet
- ACF5950 - Assignment # 7 Semester 2 2015: The Business Has The Following Opening Balances: Additional InformationDocument2 pagesACF5950 - Assignment # 7 Semester 2 2015: The Business Has The Following Opening Balances: Additional InformationkietNo ratings yet
- Enviroline 2405: Hybrid EpoxyDocument4 pagesEnviroline 2405: Hybrid EpoxyMuthuKumarNo ratings yet
- Education in America: The Dumbing Down of The U.S. Education SystemDocument4 pagesEducation in America: The Dumbing Down of The U.S. Education SystemmiichaanNo ratings yet
- Model Probabilistik: "Variable Demand and Variable Lead Time" & Konsep Service LevelDocument30 pagesModel Probabilistik: "Variable Demand and Variable Lead Time" & Konsep Service LevelVladimir Hery WijannarkoNo ratings yet
- FEM 3004 - Lab 10 Part 2editedDocument26 pagesFEM 3004 - Lab 10 Part 2editedAINA NADHIRAH BINTI A ROZEY / UPMNo ratings yet
- Chemistry Xi: Short Questions and 20% Long QuestionsDocument3 pagesChemistry Xi: Short Questions and 20% Long QuestionsSyed Nabeel HassanNo ratings yet
- Making Creams With Olive M 1000Document28 pagesMaking Creams With Olive M 1000Nicoleta Chiric0% (1)