Professional Documents
Culture Documents
FA20 234 Review 11 Amines KEY
Uploaded by
ale mOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
FA20 234 Review 11 Amines KEY
Uploaded by
ale mCopyright:
Available Formats
CHEM 234 • Organic Chemistry II • Fall 2020
Discussion activity Week 11 • Amines • KEY
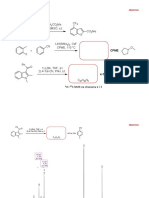
1. Predict the major product(s) for the following transformation:
NH2 F
1. NaNO2, HCl
2. HBF4
Me Me Me Me
NH2 1. NaNO2, HOAc Me
2. CuI
3. Me
SMe MgCl
SMe
cat. Pd(PPh3)4
O
1. NH3, H2,
Me cat. Ni, EtOH
2. MeI (excess), THF
3. Ag2O, H2O
4. heat
1. NaN3, DMF
O 2. H3O+ O
3. H2, Pd/C
4. MeI (xs), THF
Me 5. Ag2O, H2O, MeOH
6. heat
1 Prepared by Dr. Maria Yermolina
CHEM 234 • Organic Chemistry II • Fall 2020
Discussion activity Week 11 • Amines • KEY
1. H
N
H 2N
O O
OMe MeO
H+, MeOH
O 2. xylenes, heat N
H
O
F
HN Me 1. Br2, FeBr2 Cl
2. KOH, H2O
3. NaNO2, HCl, H2O
4. CuCl OMe
OMe
iPr
OH i-Pr
Ph 1. PCC, CH2Cl2
Ph Ph N Ph
OH 2. NH4OH, heat H
1. KOH, H2O
O O
2. Br
H
3. H2NNH2, EtOH N Me
NH
4. cat. Cp2Ln(NMe2), THF
O O
2 Prepared by Dr. Maria Yermolina
CHEM 234 • Organic Chemistry II • Fall 2020
Discussion activity Week 11 • Amines • KEY
1. NaH, DMF Ph
O
Br Ph
Me
2. H2N NH OMe
MeO N
H
cat. H+, MeOH
3. xylenes, heat
H2N Me 1. MeI (excess), THF
H iPr
2. Ag2O, H2O Me N
3. heat
4. cat. (Ph3P)2PdCl2
H 2N iPr
OH
1. heat
NMe3
2. mCPBA, CH2Cl2 OH
Me Me 3. NaCN, DMF
H 2N Me
4. LiAlH4, Et2O
5. H2O
CN
NH2 1. NaNO2, HCl, H2O H
N OMe
Br 2. CuCN, DMF
3. cat. Pd(PPh3)4, KOt-Bu
t-Bu
t-Bu
MeO NH2
3 Prepared by Dr. Maria Yermolina
CHEM 234 • Organic Chemistry II • Fall 2020
Discussion activity Week 11 • Amines • KEY
1. MeI (excess), THF
2. Ag2O, H2O
NH2 3. heat O
4. mCPBA, CH2Cl2 Ph
Me Ph
1. O
NH
S O
S O
KOH, H2O
Br 2. H2NNH2, EtOH NH2
3. HgCl2, H2O
4 Prepared by Dr. Maria Yermolina
CHEM 234 • Organic Chemistry II • Fall 2020
Discussion activity Week 11 • Amines • KEY
2. Provide the reagents to accomplish the following transformations
1. H2, Pd/C
2. NaNO2, HCl
3. NaOH
NO2 OH
O O
O O
1.
Me (excess) O
+
H (aka H2SO4 or
NH2 HN Ph
H3PO4)
iPr iPr
2. or
O O O
iPr
Ph O Ph Ph Cl
CH2Cl2
1. H2NHNPh, MeOH,
cat. H+
O 2. xylenes, heat
H
N
1. H2, Pd/C
2. Ac2O, Py OH O
NH2
3. AcCl, AlCl3
Me
O 4. KOH, H2O O
O 2. NaNO2, HCl O
3. NaOH
5 Prepared by Dr. Maria Yermolina
CHEM 234 • Organic Chemistry II • Fall 2020
Discussion activity Week 11 • Amines • KEY
1.
H 2N
O heat
HN
H Me
t-Bu 2. NaBH3CN, HOAc t-Bu
1. NaBH3CN, HOAc
2. O O
O O
Me Me
N Br N
Ph H Ph
Pd(PPh3)4 (1 mol %)
KOt-Bu, PhMe
1. NaN3, DMF
2, LiAlH4, THF
3. H3O+ H
N Me
O
Br
4 Cp2LaNMe2 (1 mol%) O
THF
or
(Ph3P)2 PdCl2 (cat)
THF
1. NH3, Ni, H2, EtOH
2.
O HN Me
Br Me
Me Me
Pd(PPh3)4 (1 mol %)
KOt-Bu, PhMe
6 Prepared by Dr. Maria Yermolina
CHEM 234 • Organic Chemistry II • Fall 2020
Discussion activity Week 11 • Amines • KEY
3. Using retrosynthetic analysis, provide a synthesis for the following molecule. Place the required
reagents next to the arrows and the necessary intermediates in the boxes provided.
H O O
Me N
Me N
Cp2LnNMe2 (1 mol %)
THF NH2, Ni, H2, EtOH
or (Ph3P)2PdOAc2 (5 mol %)
DCE
1. MeI (excess)
2. Ag2O, H2O
O 3. heat NH2 O
N Me N
NH2
O O
H2NNH2,
EtOH
H O O
Me N
Me N
Cp2LnNMe2 (1 mol %)
THF NH2, Ni, H2, EtOH
or (Ph3P)2PdOAc2 (5 mol %)
DCE
1. MeI (excess)
2. Ag2O, H2O
O 3. heat NH2 O
N Me N
NH2
O O
H2NNH2,
EtOH
7 Prepared by Dr. Maria Yermolina
CHEM 234 • Organic Chemistry II • Fall 2020
Discussion activity Week 11 • Amines • KEY
Me
Ph
1.BH3•THF, or 9BBN, THF
Br
2. NaOH, H2O2
Pd(PPh3)4 (5 mol %) 3. Swern, TEMPO
KOt-Bu, PhMe PCC or PDC oxidation
Me
H
N
H N H2NHNPh
N
CHO
H
xylenes, heat
Ph cat. H+, MeOH
Ph
O OMe
Me
NH2
Me
H3O+, heat
or
H2, Pd/C, ROH 1. KOH, H2O
2. H3O+
OH O O
heat
Me
N2 NH3
Me NaNO2, HCl
Me Cl
Me
8 Prepared by Dr. Maria Yermolina
You might also like
- Critical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsFrom EverandCritical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsNo ratings yet
- Fukuyama Group - Group Meeting Problems 2001/08/22: N N N HDocument2,429 pagesFukuyama Group - Group Meeting Problems 2001/08/22: N N N HGia PhướcNo ratings yet
- Final 211Document9 pagesFinal 211Man ChungNo ratings yet
- Exam 2 Synth 1 AnsDocument1 pageExam 2 Synth 1 AnsPhú Khang CaoNo ratings yet
- Problem Set No. 5Document12 pagesProblem Set No. 5Sofia DalisayNo ratings yet
- Paper 2 With Ans Solution ChemistryDocument15 pagesPaper 2 With Ans Solution ChemistryKushagraNo ratings yet
- Ioc 9Document3 pagesIoc 9KarthikeyanNo ratings yet
- The Ethylene Acetal A Was Also Prepared by An Alternative ApproachDocument45 pagesThe Ethylene Acetal A Was Also Prepared by An Alternative Approachbann tvNo ratings yet
- Provera Znanja 1209Document5 pagesProvera Znanja 1209ShomiNo ratings yet
- Aromatic Synthesis AnswersDocument4 pagesAromatic Synthesis AnswersLevite DeliveranceNo ratings yet
- Chem 212 Condensation Reactions 3Document1 pageChem 212 Condensation Reactions 3kevinamyNo ratings yet
- Aromatic Chemistry Assignment #3 2018-2019 ANSWERSDocument5 pagesAromatic Chemistry Assignment #3 2018-2019 ANSWERSZoe NorvilleNo ratings yet
- HW11 - Organic ChemistryDocument11 pagesHW11 - Organic ChemistryMichael NguyenNo ratings yet
- 11 01 Aminoacid 2012 ENDocument69 pages11 01 Aminoacid 2012 ENanthony.johNo ratings yet
- Quiz Chapter 12 - Alcohols From CarbonylsDocument16 pagesQuiz Chapter 12 - Alcohols From CarbonylsKaran RandhawaNo ratings yet
- PROGRESS UnsetisfactoryDocument3 pagesPROGRESS UnsetisfactoryJeffery JamesNo ratings yet
- 118c Practice Synthesis KeyDocument18 pages118c Practice Synthesis Keyapi-465421809No ratings yet
- Organic Chemistry ReactionsDocument6 pagesOrganic Chemistry ReactionsTiffany LiuNo ratings yet
- 1803 Chemistry Paper With Ans Solution EveningDocument7 pages1803 Chemistry Paper With Ans Solution EveningRahul RaiNo ratings yet
- Jee Advanced October 2021 Chemistry Paper 2 Solution - PHPDocument12 pagesJee Advanced October 2021 Chemistry Paper 2 Solution - PHPDeath RiderNo ratings yet
- Heter 0Document22 pagesHeter 0Lot AdewumilotNo ratings yet
- BCC 2019 OrgoDocument3 pagesBCC 2019 OrgoabcdefNo ratings yet
- Ca RXN CheckDocument1 pageCa RXN Checkapi-4654218090% (1)
- L-9-Opioid Alkaloids Morphine-Codeine - Thebiene and HeroinDocument15 pagesL-9-Opioid Alkaloids Morphine-Codeine - Thebiene and HeroinKavya RNo ratings yet
- Synthesis of Cyclic and Acyclic B-Amino Acids Via Chelation-Controlled 1,3-Dipolar CycloadditionDocument16 pagesSynthesis of Cyclic and Acyclic B-Amino Acids Via Chelation-Controlled 1,3-Dipolar CycloadditionNguyễn Thái DươngNo ratings yet
- CY2101Document3 pagesCY2101Prarabdha SharmaNo ratings yet
- Part - I: Practice Test-1 (Iit-Jee (Main Pattern) ) : Important InstructionsDocument22 pagesPart - I: Practice Test-1 (Iit-Jee (Main Pattern) ) : Important InstructionsKivilia EduventuresNo ratings yet
- Ingenol K. Tanino, I. Kuwajima: ActivityDocument2 pagesIngenol K. Tanino, I. Kuwajima: ActivityPercival GalahadNo ratings yet
- Nisa Slide Icnp2015Document19 pagesNisa Slide Icnp2015Yun NikNo ratings yet
- Ugi ReactionsDocument36 pagesUgi ReactionsHarjinder Singh Bhatia100% (1)
- Kaitocephalin-2 - USDocument2 pagesKaitocephalin-2 - USPercival GalahadNo ratings yet
- PS 3 ContDocument3 pagesPS 3 ContDenisse Leonoras-PatersonNo ratings yet
- (-) - Cerorubenic Acid - III Methyl Ester: L. A. Paquette, B. P. Dyck, JACS 1998, 120, 5953 - 5960Document1 page(-) - Cerorubenic Acid - III Methyl Ester: L. A. Paquette, B. P. Dyck, JACS 1998, 120, 5953 - 5960Nguyễn TấnNo ratings yet
- Redox Chemistry III: Class 9.2Document4 pagesRedox Chemistry III: Class 9.2Cao Thị Vân GiangNo ratings yet
- Synproblems AnDocument1 pageSynproblems AnEsther CruzNo ratings yet
- Massachusetts Institute of Technology 5.12, Spring 2005: Problem Set #4Document9 pagesMassachusetts Institute of Technology 5.12, Spring 2005: Problem Set #4KarthikeyanNo ratings yet
- Y2 B&SiDocument6 pagesY2 B&SiBin RenNo ratings yet
- Aromatic Problems 2013Document4 pagesAromatic Problems 2013YocobSamandrewsNo ratings yet
- Ke Li - Morphine and CodeineDocument13 pagesKe Li - Morphine and CodeinePoloGreenNo ratings yet
- Homework 2: A. HG /HG B. No /N C. So /H S D. Clo /CL E. S O /so F. Mno /MNDocument1 pageHomework 2: A. HG /HG B. No /N C. So /H S D. Clo /CL E. S O /so F. Mno /MNNam NguyenNo ratings yet
- Example Reactions From The EC Classes: Spring 2005Document3 pagesExample Reactions From The EC Classes: Spring 2005Bhadra GNo ratings yet
- 3Document3 pages3Fausto SalazarNo ratings yet
- Activity 3 Orgchem Answers Plus Xtra QuesDocument2 pagesActivity 3 Orgchem Answers Plus Xtra QuesDanesh Jemy Ann SaperoNo ratings yet
- Module1 PDFDocument122 pagesModule1 PDFAkshay MandhotraNo ratings yet
- PDF Merger 2018 05 25 16 31 25575Document393 pagesPDF Merger 2018 05 25 16 31 25575NAVEENNo ratings yet
- Proteins Consist of Amino Acids, All Natural Amino-Acids Are LDocument3 pagesProteins Consist of Amino Acids, All Natural Amino-Acids Are LMaxNo ratings yet
- Sample MCQ Organic Chemistry Sem II PSCH203 BacklogDocument4 pagesSample MCQ Organic Chemistry Sem II PSCH203 BacklogganesanneelamuruganNo ratings yet
- Practice Exam: Material Covered On Exam #3Document9 pagesPractice Exam: Material Covered On Exam #3Jaipratap SinghNo ratings yet
- Chapter 17 - Aldehydes and KetonesDocument12 pagesChapter 17 - Aldehydes and KetonesLionel MedaNo ratings yet
- PS 4Document4 pagesPS 4Denisse Leonoras-PatersonNo ratings yet
- Aromatic Compounds: 1.1 Some Useful Names 1.2 Structure 1.3 Characteristic Chemistry 1.4 Benzene ReactionsDocument6 pagesAromatic Compounds: 1.1 Some Useful Names 1.2 Structure 1.3 Characteristic Chemistry 1.4 Benzene ReactionsSarah FeyNo ratings yet
- DGT Organic Compounds C NitrogenDocument15 pagesDGT Organic Compounds C Nitrogensc5753972No ratings yet
- M.SC III SEM Scheme (Final) Organic Synthesis Jan 2017Document63 pagesM.SC III SEM Scheme (Final) Organic Synthesis Jan 2017scholarcuphy1280No ratings yet
- A B C D Answer: B, A, D, C: O O BR 1 Eq Br2 Febr3Document3 pagesA B C D Answer: B, A, D, C: O O BR 1 Eq Br2 Febr3Quốc NguyễnNo ratings yet
- Diazonium SaltsDocument20 pagesDiazonium SaltsDeepak Kumar SharmaNo ratings yet
- Chemistry of Natural Products: Dr. Mohamed RabieDocument20 pagesChemistry of Natural Products: Dr. Mohamed RabieKhalid LoveNo ratings yet
- CHEM F311 Lecture 40 Use of Aliphatic Nitro Compounds 1,2-Difunctionalised CompoundsDocument8 pagesCHEM F311 Lecture 40 Use of Aliphatic Nitro Compounds 1,2-Difunctionalised Compoundsliving luxuriousNo ratings yet
- Problem Set 9 KEYDocument3 pagesProblem Set 9 KEYCARLOS ALBERTO OSORIO MARTINEZNo ratings yet
- Om en A: F in Ite D Iffe Ren Ce-B Ase DN Um Eri Ca L M Eth Od SDocument426 pagesOm en A: F in Ite D Iffe Ren Ce-B Ase DN Um Eri Ca L M Eth Od SPrateek Kumar PandeyNo ratings yet
- Research 9: Activity 4: Background of The StudyDocument7 pagesResearch 9: Activity 4: Background of The StudyPhilip AmelingNo ratings yet
- MT4400 STRG Flo Amp ValveDocument7 pagesMT4400 STRG Flo Amp ValveBrian Careel0% (1)
- 93c3 Document 3Document14 pages93c3 Document 3NONON NICOLASNo ratings yet
- Term Test Pointers For Review - 1st TermDocument2 pagesTerm Test Pointers For Review - 1st Termjessica holgadoNo ratings yet
- Cooling and Sealing Air System: Gas Turbine Training ManualDocument2 pagesCooling and Sealing Air System: Gas Turbine Training ManualVignesh SvNo ratings yet
- PC-ABS Bayblend FR3010Document4 pagesPC-ABS Bayblend FR3010countzeroaslNo ratings yet
- MLAB 3 - BoilerDocument3 pagesMLAB 3 - BoilerReden LopezNo ratings yet
- Interjections NotesDocument2 pagesInterjections NotesKanna ImuiNo ratings yet
- (English (Auto-Generated) ) Intraday Trading On Nifty (2nd September, 2021) 8 Lakhs Profit Shreyas Bandi Trade Ideas Live (DownSub - Com)Document41 pages(English (Auto-Generated) ) Intraday Trading On Nifty (2nd September, 2021) 8 Lakhs Profit Shreyas Bandi Trade Ideas Live (DownSub - Com)YaaroNo ratings yet
- Xgenus X-Ray PDFDocument61 pagesXgenus X-Ray PDFAli NuriNo ratings yet
- The Extension Delivery SystemDocument10 pagesThe Extension Delivery SystemApril Jay Abacial IINo ratings yet
- Module 3: Literature Review and CitationDocument3 pagesModule 3: Literature Review and CitationLysss EpssssNo ratings yet
- Beamforming For 4.9G/5G Networks: Exploiting Massive MIMO and Active Antenna TechnologiesDocument12 pagesBeamforming For 4.9G/5G Networks: Exploiting Massive MIMO and Active Antenna TechnologiesAymen Ben zinebNo ratings yet
- Hemo TecaDocument17 pagesHemo TecaMafer PilcoNo ratings yet
- Hamming Code - Error Detection Aim: AlgorithmDocument12 pagesHamming Code - Error Detection Aim: Algorithmkrithikgokul selvamNo ratings yet
- C7 On-Highway Engine Electrical System: Harness and Wire Electrical Schematic SymbolsDocument2 pagesC7 On-Highway Engine Electrical System: Harness and Wire Electrical Schematic SymbolsFeDe Aavina Glez100% (3)
- Rivers and Their Origin (Top MCQ)Document24 pagesRivers and Their Origin (Top MCQ)Anil Yadav100% (1)
- IMG - 0009 Thermodynamic Lecture MRCDocument1 pageIMG - 0009 Thermodynamic Lecture MRCBugoy2023No ratings yet
- Chapter 6 SBLDocument4 pagesChapter 6 SBLbrave manNo ratings yet
- Entropy Equation For A Control VolumeDocument12 pagesEntropy Equation For A Control VolumenirattisaikulNo ratings yet
- Application Bright Ideas Education Grant Program For TeachersDocument6 pagesApplication Bright Ideas Education Grant Program For Teachersapi-320983699No ratings yet
- Chapter 15 - Leukocyte Migration and Inflammation - The IS Relies Upon The Continual Circulation of Leukocytes Through The BodyDocument12 pagesChapter 15 - Leukocyte Migration and Inflammation - The IS Relies Upon The Continual Circulation of Leukocytes Through The BodyEmad ManniNo ratings yet
- Summative 1Document4 pagesSummative 1Nean YsabelleNo ratings yet
- IES 2001 - I ScanDocument20 pagesIES 2001 - I ScanK.v.SinghNo ratings yet
- Instruction Manual: Slit Lamp Bon SL-EDocument20 pagesInstruction Manual: Slit Lamp Bon SL-EVladimir LevchenkoNo ratings yet
- DCS Ground Charts v350Document34 pagesDCS Ground Charts v350lkjsdflkjNo ratings yet
- (Ebook) Reliability and Risk Issues in Large Scale Safety-Critical Digital Control Systems, Springer 2009Document314 pages(Ebook) Reliability and Risk Issues in Large Scale Safety-Critical Digital Control Systems, Springer 2009Lake HouseNo ratings yet
- Is There Any Way To Download The Whole Package of Asphalt 8 Airborne So That I Can Install It On Any Android Device Without An Internet Connection - QuoraDocument4 pagesIs There Any Way To Download The Whole Package of Asphalt 8 Airborne So That I Can Install It On Any Android Device Without An Internet Connection - QuoraMounir2105No ratings yet
- Effect of Liquidity Risk On Performance of Deposit Money Banks in NigeriaDocument6 pagesEffect of Liquidity Risk On Performance of Deposit Money Banks in NigeriaEditor IJTSRDNo ratings yet