Professional Documents
Culture Documents
Aspergillin PZ, A Novel Isoindole-Alkaloid From Aspergillus Awamori
Uploaded by
Alec LiuOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Aspergillin PZ, A Novel Isoindole-Alkaloid From Aspergillus Awamori
Uploaded by
Alec LiuCopyright:
Available Formats
VOL.55 NO. 8, AUG.2002 THE JOURNAL OF ANTIBIOTICS pp.
693-695
Aspergillin PZ, a Novel Isoindole-alkaloid from Aspergillus awamori
YI ZHANG,TAOWANG,YUEHUPEI*, HUIMINGHUA and BAOMINFENG
College of Traditional Chinese Medicines, Shenyang Pharmaceutical University,
Shenyang 110016, China
(Received for publication December 25, 2001)
Aspergillin PZ was obtained from the fermentation of Aspergillus awamori (Nakazawa) by
activity-guided fractionation and purification. Its structure was elucidated on the basis of
spectral data, especially by 2D NMR, and finally confirmed by an X-ray analysis. It could
induce conidia of P. oryzae to deform moderately.
In the course of screening anti-tumor and anti-fungal 173.1, 211.6). Among these signals, δ 127.5 (C-6) and
agents from secondary metabolites of soil fungi using 138.7 (C-5) indicated the presence of a trisubstituted double
Pyricularia oryzae as the primary screening model, we bond group, δ 211.6 and 173.1 suggested the presences of a
found that the fermentation of Aspergillus awamori carbonyl group and an amide carbonyl group, respectively.
In the 1H-1H COSY spectrum, the correlations of δ 1.70
(Nakazawa) could induce mycelia of P oryzae to geminate
from conidia1). Aspergillin PZ was obtained from the (H-2') to δ 0.86 (2'-CH3, H3-3') and 1.20 (HA-1'); δ 2.90
fermentation broth by activity-guided fractionation, which (H-3) to δ 1.20 (HA-1'), 1.48 (HB-1'), 2.38 (H-3a) and 8.00
posses a new carbon skeleton. Its structure was elucidated (2-NH); δ 2.47 (H-4) to δ 1.07 (4-CH3) and 2.38 (H-3a)
on the basis of spectral data, especially by 2D NMR, and indicated the fragment A as shown (Fig. 1). The moiety B
finally confirmed by an X-ray analysis. In this paper, the was deduced by correlations of δ 1.51 (HA-9) to δ 1.32
production, isolation, biological activity, and structural (HA-8), 1.79 (HB-9), 1.81 (HB-8) and 3.38 (H-10); δ 3.38
elucidation are reported. (H-10) to δ 3.50 (H-11), 4.49 (OH). Furthermore, the
The fungus A. awamori was isolated from a soil sample correlations of δ 2.67 (H-11a) to δ2.29 (HA-12), 2.56 (HB-
collected from Heibei province, China. It was cultured 12) and 2.77 (H-6b); δ 2.50 (H-6a) to δ 2.77 (H-6b) and
using Sabourad's medium (Polypeptone (10g/liter), NaCl 5.44 (H-6); δ 5.44 (H-6) to 1.72 (5-CH3) suggested the
(5g/liter), Glucose (40g/liter)) at 28℃ and 260rpm for presence of fragment C (Fig. 1).
120 hours. The fermentation (7.0 liters) was partitioned The long range 1H-13C correlations of δ 1.10 (3H, s, 7α-
with ethyl acetate. CH3) to δ 81.4 (C-7), 34.7 (C-8) and 42.4 (C-6b); δ 3.50
The EtOAc extract (15g) was chromatographed on a (H-11) to δ 42.6 (C-12); δ (2.29 and 2.56, each 1H, m, H-
silica gel column (300g, 8cm×40cm) with a solvent 12) to δ 211.6; δ 1.07 (4β-CH3) to δ 138.7 (C-5); δ 8.00
system of CHCl3-MeOH (100:2) and further recrystalized (2-NH) to δ 173.1 (C-1) and 63.9 (C-13a) in HMBC
in CHCl3-MeOH (1:10), to give aspergillin PZ (100mg). spectrum (Fig. 2) led to determination of the connectivities
Aspergillin PZ was obtained as colorless square crystal. of three fragments (A, B and C, Fig. 1) in the molecule. All
Its molecular formula was inferred by HREI-MS (m/z protons and carbons were assigned on the basis of 1H-1H
401.2571) as C24H35NO4.The 13C NMR and DEPT of it COSY, HMQC and HMBC spectra (Table 1). Aspergillin
displayed five methyls signals (δ 13.4, 19.9, 21.4, 23.3, PZ was designated as 3β, 3aα, 4α, 6aα-tetrahydro-10α-
23.9), four methylenes (δ 24.7, 34.7, 42.6, 48.1), nine hydroxy-4β,5,7α-trimethyl-3α-(2-methylpropyl)-[cis-
methines (δ 24.0, 34.3, 35.8, 38.7, 42,4, 51.1, 51.6, 65.2, transoid-trans-(12-oxatricyclo[6.3.1.02,7])]dodeca[4,3-
83.4), 2 quaternary carbons (δ 63.9, 81.4), 2 olefinic d]isoindole-1 (H), 13-dione. The structure was finally
carbons (δ 127.5, 138.7) and two carbonyl carbons (δ verified by an X-ray analysis (Fig. 3). The skeletal structure
* Corresponding author: yuehpei@online.ln.cn
694 THE JOURNAL OF ANTIBIOTICS AUG. 2002
Fig. 1. Structure fragments of aspergillin PZ, and 1H-1H COSY of it.
Fig. 2. Key HMBC correlations of Fig. 3. ORTEP drawing of aspergillin PZ.
asepergillin PZ.
Table 1. NMR data of aspergillin PZ.
1H NMR (300MHz, DMSO-d6), 13CNMR (75MHz, DMSO-d6)
*: data of them can be exchanged.
VOL. 55 NO. 8 THE JOURNAL OF ANTIBIOTICS 695
of aspochalasin C was reported to be very similar to Acknowledgments
aspergillin PZ2). The addition of 18-OH of the former
The present work was supported by Foundation of State Key
compound followed by cyclization would give the latter
Basic Research and Development Project (G1998051100),
one. The relative stereochemistry of C-3, C-3a, C-4 and Beijing. The strain was identified by Prof. SJ WANG of
C-13a of aspergillin PZ was related to the counterpart Institute of Applied Ecology Academia Sinica, Shenyang,
of aspochalasin C. China.
In previous research, we found that the analogs of
aspergillin PZ isolated from aspergillum genus could References
induce conidia of P. oryzae to deform moderately.
1) KOBAYASHI, H.; M. NAMIKOSHI, T. YOSHIMOTO & T.
Furthermore, they had activity against cancer cells such as YOKOCHI:A screening method for antimitotic and
MH-60 and HL-60, which indicated the connection antifungal substances using conidia of Pyricularia
between anti-tumor and anti-P. oryzae3) activities. oryzae, modification and application to tropical marine
fungi. J. Antibiotics 49: 873-879, 1996
Pharmacological research indicated aspergillin PZ could
2) KATARINA, N. L.& D. MAX: Stoffwechselprodukte von
induce morphological deformation of conidia of P. oryzae Mikroorganismen. Helv. Chim. Acta 65: 1426-1427,
strongly at 0.089μM. This evidence suggested that the 1982
compound had activity to tumor cell. Moreover, the content 3) FANG, F.; H. UI, K. SHIOMI, R. MASUMA, Y. YAMAGUCHI,
C. G. ZHANG,X. W. ZHANG,Y. TANAKA& S. OMURA:
of it in EtOAc extracts was 0.833%, which was very high.
Two new components of the aspochalasins produced by
So we can obtain it by improving the fungus if it has Aspergillus sp. J. Antibiotics 50: 919-925, 1997
activities to cancer cells. Further pharmacological research
of it is now under way in our laboratory.
You might also like
- Nepetaefolins A J, Cytotoxic Chinane and Abietane Diterpenoids From Caryopteris NepetaefoliaDocument8 pagesNepetaefolins A J, Cytotoxic Chinane and Abietane Diterpenoids From Caryopteris NepetaefoliaAlexis CushicondorNo ratings yet
- Wei-Dong Xie, Xia Li, and Kyung Ho RowDocument8 pagesWei-Dong Xie, Xia Li, and Kyung Ho Rowseptodrasta123No ratings yet
- A Benzopyrroloisoquinoline Alkaloid From Ficus Fistulosa PDFDocument3 pagesA Benzopyrroloisoquinoline Alkaloid From Ficus Fistulosa PDFBianca HidayatNo ratings yet
- np060194hDocument4 pagesnp060194hAldo RodarteNo ratings yet
- 2001 Isolation of Symlandine From The Roots of Common Comfrey (Symphytum Usin Coutercurrent ChromDocument3 pages2001 Isolation of Symlandine From The Roots of Common Comfrey (Symphytum Usin Coutercurrent ChromBackup MelacromNo ratings yet
- Polyoxygenated Cyclohexene Derivatives From Ellipeiopsis CherrevensisDocument7 pagesPolyoxygenated Cyclohexene Derivatives From Ellipeiopsis CherrevensisamensetNo ratings yet
- (A) - (7) Kuo1995Document4 pages(A) - (7) Kuo1995yến ngô bảoNo ratings yet
- Shorea Hemsleyana InggDocument5 pagesShorea Hemsleyana InggKhairie Prazoeber HerkaNo ratings yet
- 1 s2.0 S0040402013009824 MainDocument5 pages1 s2.0 S0040402013009824 MainLizhy PedrazaNo ratings yet
- Pregnane Glycosides From Sansevieria Trifasciata: OH J,, K J - . JDocument5 pagesPregnane Glycosides From Sansevieria Trifasciata: OH J,, K J - . JRommelAnastacioNo ratings yet
- Nozaki 2007Document3 pagesNozaki 2007AnaRafaelaSilvaNo ratings yet
- Antibacterial Clerodane Diterpenes from GoldenrodDocument6 pagesAntibacterial Clerodane Diterpenes from GoldenrodNicoleta GrosuNo ratings yet
- PHYTOCHEMISTRY OF PIPER CRASSINERVIUMDocument6 pagesPHYTOCHEMISTRY OF PIPER CRASSINERVIUMAndre HaroNo ratings yet
- Diterpenes From Dysoxylum Lukii MerrDocument4 pagesDiterpenes From Dysoxylum Lukii MerrJuan AspilcuetaNo ratings yet
- Ali 2009Document5 pagesAli 2009Long ManNo ratings yet
- Chemical Constituents From The Colombian Medicinal Plant Maytenus LaevisDocument6 pagesChemical Constituents From The Colombian Medicinal Plant Maytenus LaevisCarlos Sopán BenauteNo ratings yet
- A Novel 2-Hydroxyflavanone From Collinsonia CanadensisDocument3 pagesA Novel 2-Hydroxyflavanone From Collinsonia CanadensisAuroTest. deNo ratings yet
- New phenylethanoid glycosides from Picria felterraeDocument5 pagesNew phenylethanoid glycosides from Picria felterraePahlawan SagasatuNo ratings yet
- Komponen Pisialis AngulataDocument7 pagesKomponen Pisialis Angulatanurhayati novitaNo ratings yet
- Amphimedonoic Acid and Psammaplysene E Novel Brominated A - 2017 - TetrahedronDocument4 pagesAmphimedonoic Acid and Psammaplysene E Novel Brominated A - 2017 - TetrahedronC1Muhammad Iqbal FarozinNo ratings yet
- ARTICLE - A New Triterpenoid Saponin From Ononis SpinosaDocument5 pagesARTICLE - A New Triterpenoid Saponin From Ononis SpinosaKozi UfiaNo ratings yet
- Biflavonoid - 1999 - J Nat Prod. 62 p1668Document4 pagesBiflavonoid - 1999 - J Nat Prod. 62 p1668Khiem Thai Ba BaoNo ratings yet
- Teulade 1983Document5 pagesTeulade 1983Francesco AdingraNo ratings yet
- Two New Flavonoids From Centella Asiatica PDFDocument5 pagesTwo New Flavonoids From Centella Asiatica PDFJ C Torres FormalabNo ratings yet
- harinantenaina2007Document3 pagesharinantenaina2007xkhanhxkhanhNo ratings yet
- Bangladesh Journal of Pharmacology: Volume: 14 Number 4 Year 2019Document3 pagesBangladesh Journal of Pharmacology: Volume: 14 Number 4 Year 2019zainab jehangirNo ratings yet
- Diarylheptanoids From The Rhizomes of Zingiber OfficinaleDocument4 pagesDiarylheptanoids From The Rhizomes of Zingiber OfficinaleTung VuNo ratings yet
- 02 Rothman NiaDocument4 pages02 Rothman NiaNuo PinkoNo ratings yet
- ZNB 2009 0913Document7 pagesZNB 2009 0913Tiara WelchNo ratings yet
- A New Ent-Clerodane Diterpene From The Earial Parts of Baccharis Gaudichaudiana (2003)Document3 pagesA New Ent-Clerodane Diterpene From The Earial Parts of Baccharis Gaudichaudiana (2003)TàiNguyễnThànhNo ratings yet
- A Diels-Alder-Type Adduct From Artocarpus HeterophyllusDocument3 pagesA Diels-Alder-Type Adduct From Artocarpus HeterophyllusiswanNo ratings yet
- Trichoderma Reesei: Trichodermatides A D, Novel Polyketides From The Marine-Derived FungusDocument4 pagesTrichoderma Reesei: Trichodermatides A D, Novel Polyketides From The Marine-Derived FunguspoonamsharmapoornimaNo ratings yet
- Agastaquinone, A New Cytotoxic DiterpenoidDocument4 pagesAgastaquinone, A New Cytotoxic Diterpenoidela.sofiaNo ratings yet
- Terpenoids D. LinearisDocument6 pagesTerpenoids D. LinearisCah LilinNo ratings yet
- Allergy-Preventive Phenolic Glycosides From Populus SieboldiiDocument3 pagesAllergy-Preventive Phenolic Glycosides From Populus SieboldiiCarmen Yuliana GutierrezNo ratings yet
- Phosphorus, Sulfur, and SiliconDocument6 pagesPhosphorus, Sulfur, and SiliconDrBonny PatelNo ratings yet
- Đơn đỏ-bbb0776Document5 pagesĐơn đỏ-bbb0776Mai Anh NguyễnNo ratings yet
- 1057Document5 pages1057Amer KasidehNo ratings yet
- Fusidic Acid From Corchorus Aestuans L.Document8 pagesFusidic Acid From Corchorus Aestuans L.Dr. Ramadevi DevarakondaNo ratings yet
- Kuznetsov 2007Document3 pagesKuznetsov 2007CriztIan GgomesNo ratings yet
- Yang2016 THYMOQUINOLDocument23 pagesYang2016 THYMOQUINOLPlant VietNo ratings yet
- J. Nat. Prod., 2000, 63 (4), 436-440Document6 pagesJ. Nat. Prod., 2000, 63 (4), 436-440Casca RMNo ratings yet
- SIX NEW HETEROCYCLIC STILBENE OLIGOMERS FROM SHOREA HEMSLEYANA STEM BARKDocument12 pagesSIX NEW HETEROCYCLIC STILBENE OLIGOMERS FROM SHOREA HEMSLEYANA STEM BARKKhairie Prazoeber HerkaNo ratings yet
- Khuthier 1987Document4 pagesKhuthier 1987Brem BalazsNo ratings yet
- A New Tropane Alkaloid and Other Constituents of Erytorxylum RimosumDocument4 pagesA New Tropane Alkaloid and Other Constituents of Erytorxylum RimosumGustavo RuizNo ratings yet
- Isolation of (-) - Epicatechin From Trichilia Emetica Whole SeedsDocument5 pagesIsolation of (-) - Epicatechin From Trichilia Emetica Whole Seedstuấn anhNo ratings yet
- 1 s2.0 S003194220200184X MainDocument4 pages1 s2.0 S003194220200184X MainanneNo ratings yet
- NH2AZIDocument3 pagesNH2AZIdelfin000No ratings yet
- Huang 2008Document8 pagesHuang 2008pratikxeo3No ratings yet
- Ezeoke2018 Alkaloid e TectoriusDocument7 pagesEzeoke2018 Alkaloid e TectoriusRahayu UtamiNo ratings yet
- Flavonoids and Phenolic Acids From The Aerial Parts of Alyssum Alyssoides L. (Brassicaceae)Document3 pagesFlavonoids and Phenolic Acids From The Aerial Parts of Alyssum Alyssoides L. (Brassicaceae)Diamanto LazariNo ratings yet
- Elliptica: A, b,1 A, 1 C A, B A, B A A A A B, ADocument7 pagesElliptica: A, b,1 A, 1 C A, B A, B A A A A B, ALuz Marina Peña MorajxkfjjkkzkfNo ratings yet
- Antraquinonas LeituraDocument5 pagesAntraquinonas LeituraAdriane CardozoNo ratings yet
- Untul Pamantului ArticoleDocument2 pagesUntul Pamantului ArticoleRoxana FloreaNo ratings yet
- OH O O: Griffiths, Russell Jon, PCT Int. Appl., 2013124682, 29 Aug 2013Document3 pagesOH O O: Griffiths, Russell Jon, PCT Int. Appl., 2013124682, 29 Aug 2013kasun1237459No ratings yet
- Jurnal 34 3Document3 pagesJurnal 34 3Isra Tri HardiantiNo ratings yet
- Andropanolide and Isoandrographolide, Minor Diterpenoids From Andrographis Paniculata: Structure and X-Ray Crystallographic AnalysisDocument3 pagesAndropanolide and Isoandrographolide, Minor Diterpenoids From Andrographis Paniculata: Structure and X-Ray Crystallographic AnalysisShimul HalderNo ratings yet
- Phloroglucinol Derivatives From Three Australian Marine Algae of The Genus ZonarzaDocument3 pagesPhloroglucinol Derivatives From Three Australian Marine Algae of The Genus ZonarzaGoretti ArvizuNo ratings yet
- Chalcona e Diidrochalcona PDFDocument3 pagesChalcona e Diidrochalcona PDFamensetNo ratings yet
- The Chemistry of Natural Products: 6: Plenary Lectures Presented at the Sixth International Symposium on the Chemistry of Natural ProductsFrom EverandThe Chemistry of Natural Products: 6: Plenary Lectures Presented at the Sixth International Symposium on the Chemistry of Natural ProductsNo ratings yet
- 22 Needs Analysis QuestionnaireDocument1 page22 Needs Analysis QuestionnaireAlec LiuNo ratings yet
- 12 Phrasal SnapDocument2 pages12 Phrasal SnapAlec LiuNo ratings yet
- C5T1S3 - Management Diploma CoursesDocument2 pagesC5T1S3 - Management Diploma CoursesAlec LiuNo ratings yet
- 18 Phone Me Bingo CardsDocument1 page18 Phone Me Bingo CardsAlec LiuNo ratings yet
- 13 Learners Word ListDocument1 page13 Learners Word ListAlec LiuNo ratings yet
- 4 I Am Getting Used To ItDocument2 pages4 I Am Getting Used To ItAlec LiuNo ratings yet
- C5T2S2 - Bicycles For The WorldDocument2 pagesC5T2S2 - Bicycles For The WorldAlec LiuNo ratings yet
- C5T1S3 - Management Diploma CoursesDocument2 pagesC5T1S3 - Management Diploma CoursesAlec LiuNo ratings yet
- BBC Two - Trust Me, I'm A Doctor, Series 8, Episode 1 - Can Learning A New Language Boost Your Brain?Document2 pagesBBC Two - Trust Me, I'm A Doctor, Series 8, Episode 1 - Can Learning A New Language Boost Your Brain?Alec LiuNo ratings yet
- C5T1S1 - Dreamtime Travel AgencyDocument2 pagesC5T1S1 - Dreamtime Travel AgencyAlec Liu100% (2)
- Speakout Writing Extra Intermediate Unit 01 PDFDocument1 pageSpeakout Writing Extra Intermediate Unit 01 PDFJon WeaverNo ratings yet
- Interviews Worksheet PDFDocument4 pagesInterviews Worksheet PDFАмир КувватовNo ratings yet
- C5T2S1 - Library InformationDocument1 pageC5T2S1 - Library InformationAlec LiuNo ratings yet
- C5T1S4 - Management of Personal FinancesDocument1 pageC5T1S4 - Management of Personal FinancesAlec LiuNo ratings yet
- Results of The Rumphius Biohistorical Expedition To Ambon (1990)Document24 pagesResults of The Rumphius Biohistorical Expedition To Ambon (1990)Alec LiuNo ratings yet
- C5T1S2 - 'Buyer Beware' ProgrammeDocument1 pageC5T1S2 - 'Buyer Beware' ProgrammeAlec LiuNo ratings yet
- A Revision of T H E Genus Lesson (Octocorallia, Alcyonacea)Document113 pagesA Revision of T H E Genus Lesson (Octocorallia, Alcyonacea)Alec LiuNo ratings yet
- Providencia: An Island With A Sea of Seven Colours'Document10 pagesProvidencia: An Island With A Sea of Seven Colours'Alec LiuNo ratings yet
- Making Food Crops That Feed Themselves - BBC NewsDocument10 pagesMaking Food Crops That Feed Themselves - BBC NewsAlec LiuNo ratings yet
- Speakout DVD Extra Intermediate Unit 01Document1 pageSpeakout DVD Extra Intermediate Unit 01Shwe Maw KunNo ratings yet
- 'How Learning A Foreign Language Changed My Life' - BBC NewsDocument8 pages'How Learning A Foreign Language Changed My Life' - BBC NewsAlec LiuNo ratings yet
- Criss Cross Puzzle B11 T1 P1Document1 pageCriss Cross Puzzle B11 T1 P1Alec LiuNo ratings yet
- Growing Higher - New Ways To Make Vertical Farming Stack Up - Science and Technology - The EconomistDocument5 pagesGrowing Higher - New Ways To Make Vertical Farming Stack Up - Science and Technology - The EconomistAlec LiuNo ratings yet
- Finding Key WordsDocument1 pageFinding Key WordsAlec LiuNo ratings yet
- DiceDocument1 pageDiceAlec LiuNo ratings yet
- L1 U2 AnswerDocument5 pagesL1 U2 AnswerAlec Liu100% (2)
- B1 Writing U2Document1 pageB1 Writing U2Alec LiuNo ratings yet
- L1 U2 WorkbookDocument38 pagesL1 U2 WorkbookAlec Liu100% (1)
- How our sweet tooth is hurting usDocument37 pagesHow our sweet tooth is hurting usAlec Liu71% (14)
- XII Computer Science Pre-Board Exam Answer KeyDocument13 pagesXII Computer Science Pre-Board Exam Answer KeySanjay Dani100% (1)
- Gen Math - 2st Quarter 45Document2 pagesGen Math - 2st Quarter 45John Rey CantoriaNo ratings yet
- Flow Diagram, Equipment and Tag IdentificationDocument24 pagesFlow Diagram, Equipment and Tag Identificationmkpq100% (1)
- RRB Clerk Prelims Day - 13 e (1) 168804293277Document27 pagesRRB Clerk Prelims Day - 13 e (1) 168804293277sahilNo ratings yet
- Bios Cmos MemoryDocument23 pagesBios Cmos MemoryEmman BalicoNo ratings yet
- Teradata 13.10 FeaturesDocument43 pagesTeradata 13.10 FeaturesLansa PhillipNo ratings yet
- Eurocode Assessment For BridgesDocument12 pagesEurocode Assessment For BridgesrenandNo ratings yet
- Surveying NotesDocument7 pagesSurveying NotesTrina SambasNo ratings yet
- PS3 Prompt Explained With Examples in LinuxDocument2 pagesPS3 Prompt Explained With Examples in LinuxSaka KelyNo ratings yet
- Soft ComputingDocument38 pagesSoft ComputingGayatri Dilip JuwatkarNo ratings yet
- Glass Balustrade Structural CalculationDocument11 pagesGlass Balustrade Structural CalculationMONERA d.o.o.No ratings yet
- Brochure Allweiler AllfuelDocument12 pagesBrochure Allweiler AllfuelbalramkinageNo ratings yet
- Introduction To Human Anatomy and Physiology: Joher B. Mendez, JR., R.N., M.DDocument65 pagesIntroduction To Human Anatomy and Physiology: Joher B. Mendez, JR., R.N., M.DJoherNo ratings yet
- Heat Technology: Chapter 10 Section 4Document23 pagesHeat Technology: Chapter 10 Section 4thegedusNo ratings yet
- Section I Introduction To Magnetic Particle InspectionDocument130 pagesSection I Introduction To Magnetic Particle Inspectionhvu9743No ratings yet
- Machine Design-I and II Lecture NotesDocument37 pagesMachine Design-I and II Lecture NotesAzeem MohammadNo ratings yet
- Mechanical Seal CatalogDocument172 pagesMechanical Seal Catalogcristian villegasNo ratings yet
- A13-2019-LR-Predicting Supply Chain Risks Using Machine Learning The Trade-Off Between Performance and Interpretability PDFDocument12 pagesA13-2019-LR-Predicting Supply Chain Risks Using Machine Learning The Trade-Off Between Performance and Interpretability PDFVỹ PhạmNo ratings yet
- Rank and Nullity TheoremDocument6 pagesRank and Nullity TheoremsdfsdfNo ratings yet
- Soal Am 2023 - Bahasa InggrisDocument13 pagesSoal Am 2023 - Bahasa Inggrisfarukikamal48No ratings yet
- McqsDocument14 pagesMcqschandramohan muruganNo ratings yet
- Is Is at At: P,/P P, P,/PDocument4 pagesIs Is at At: P,/P P, P,/PNaqqash SajidNo ratings yet
- Numerical Method For Engineers-Chapter 14Document8 pagesNumerical Method For Engineers-Chapter 14MrbudakbaekNo ratings yet
- JVC RX Es1slDocument20 pagesJVC RX Es1slvideosonNo ratings yet
- Reaction KineticsDocument26 pagesReaction Kineticssuhaila ramleyNo ratings yet
- MVB-UART DatasheetDocument24 pagesMVB-UART DatasheetmrezafarahrazNo ratings yet
- Preparatory Guidance From Infosys - SP and DSE RolesDocument9 pagesPreparatory Guidance From Infosys - SP and DSE RolesSONALI MA80% (5)
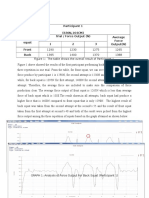
- Result: Participant 1 (630N, 164CM) Type of Squat Trial / Force Output (N) Average Force Output (N) 1 2 3 Front BackDocument5 pagesResult: Participant 1 (630N, 164CM) Type of Squat Trial / Force Output (N) Average Force Output (N) 1 2 3 Front BackpatenggNo ratings yet