Professional Documents
Culture Documents
3shape Implant Studio Drilling Protocol
Uploaded by
nha khoa NHƯ NGỌCOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
3shape Implant Studio Drilling Protocol
Uploaded by
nha khoa NHƯ NGỌCCopyright:
Available Formats
Contact information:
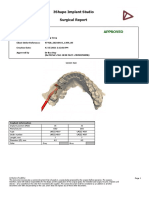
3Shape Implant
Studio
Drilling Protocol
Order Details
Patient Name: Tung Vu Thanh
Client Order Reference: 97728_20210427_1625_55 (1)
Creation Date: 4/27/2021 5:06:18 PM
Created by: Dr Bao Duy
(0e7819ef-c561-4b98-9627-c7bf8025089b)
*1=Note: Please check the required final drill length against the suggested minimum drill length for your chosen implants. The final drill length depends on the chosen sleeve type and instructions for use for
the relevant surgical protocol defined by your implant choice. E.g., for fully guided sleeves additional values might need to be added like a potentially used spoon or extended drill depth.
Implant information
Implant position (FDI) 37
Manufacturer DIO
Type UF(II) 4008
Order number UF(II) 4008
Length, mm 8.5
Diameter (Ø), mm 4
Color Red
Sleeve information
Name DIO GS 53
Type Fully guided
Order number GS 53
Offset, mm 9
Color Blue
Drill information
Minimum drill length 17.5
Limitation of Liability: Page 1
This instruction incorporates a custom document that is based on a surgical plan proposed by the surgeon before operation. The surgeon, therefore, takes full medical responsibility for
the design and the application of the surgical guide, the intended used surgical tray kit, implants and sleeves – all as specified on the order form received by the supplier. The custom
document shall be considered as an addition to all other documents sent with and pertaining to the case, and it does not replace any of those other documents.
Implant information
Implant position (FDI) 47
Manufacturer DIO
Type UF(II) 4008
Order number UF(II) 4008
Length, mm 8.5
Diameter (Ø), mm 4
Color Red
Sleeve information
Name DIO GS 53
Type Fully guided
Order number GS 53
Offset, mm 9
Color Blue
Drill information
Minimum drill length 17.5
Limitation of Liability: Page 2
This instruction incorporates a custom document that is based on a surgical plan proposed by the surgeon before operation. The surgeon, therefore, takes full medical responsibility for
the design and the application of the surgical guide, the intended used surgical tray kit, implants and sleeves – all as specified on the order form received by the supplier. The custom
document shall be considered as an addition to all other documents sent with and pertaining to the case, and it does not replace any of those other documents.
You might also like
- Cervical RibsDocument4 pagesCervical RibsshilusharmaNo ratings yet
- RISK MANAGEMENT Report ExampleDocument4 pagesRISK MANAGEMENT Report Examplepatricia colin100% (1)
- Engineering - Bulletin - Calculating Thread StrengthDocument2 pagesEngineering - Bulletin - Calculating Thread StrengthrobigedNo ratings yet
- Neodent Guided Kit PDFDocument24 pagesNeodent Guided Kit PDFBehdad JavdanNo ratings yet
- International Patient Safety Goals (Ipsg)Document9 pagesInternational Patient Safety Goals (Ipsg)Shibin100% (1)
- Minimally Invasive Dental Implant SurgeryFrom EverandMinimally Invasive Dental Implant SurgeryDaniel R. CullumNo ratings yet
- INGG1000Document9 pagesINGG1000skdewediNo ratings yet
- 2 5350461062384715429 PDFDocument322 pages2 5350461062384715429 PDFLaurie100% (2)
- Ophthalmic Dictionary and Vocabulary Builder For Eye Care ProfessionalsDocument431 pagesOphthalmic Dictionary and Vocabulary Builder For Eye Care ProfessionalsPutri Rosalina TamzilNo ratings yet
- US20190376758A1Document8 pagesUS20190376758A1Jean DelaronciereNo ratings yet
- BFD-180-570 DN65 GBDocument25 pagesBFD-180-570 DN65 GBalexander100% (3)
- Protaper TechniqueDocument14 pagesProtaper TechniquePiyush Varshney100% (1)
- Senior OrificeDocument7 pagesSenior OrificeamarnethaNo ratings yet
- Exocad Surgical Plan ModelDocument2 pagesExocad Surgical Plan ModelFernando Pinto AlvarezNo ratings yet
- 3shape Implant Studio Drilling Protocol: Contact InformationDocument2 pages3shape Implant Studio Drilling Protocol: Contact Informationnha khoa NHƯ NGỌCNo ratings yet
- Drilling ProtocolDocument1 pageDrilling ProtocolEdwin Lupaca ArocutipaNo ratings yet
- SurgicalReport UnapprovedDocument5 pagesSurgicalReport Unapprovedpyxk5c2yzmNo ratings yet
- 3shape Implant Studio Surgical Report: ApprovedDocument3 pages3shape Implant Studio Surgical Report: Approvednha khoa NHƯ NGỌCNo ratings yet
- Surgical ReportDocument8 pagesSurgical ReportTiago Oliveira ImperiortoNo ratings yet
- 3shape Implant Studio Surgical Report: ApprovedDocument3 pages3shape Implant Studio Surgical Report: Approvednha khoa NHƯ NGỌCNo ratings yet
- NomogramDocument1 pageNomogramSPOSI MTAurangabadNo ratings yet
- 09 Nail-Instruction For UseDocument4 pages09 Nail-Instruction For UseDiana Rodriguez ParodiNo ratings yet
- Multipoint Rod Extensometer: User ManualDocument20 pagesMultipoint Rod Extensometer: User ManualAndres MejiaNo ratings yet
- End Cover - D-PTTB 4-TG - 3211918: Key Commercial DataDocument3 pagesEnd Cover - D-PTTB 4-TG - 3211918: Key Commercial DataArvin PNo ratings yet
- Implant Details: Plan: Position: 33 SleeveDocument2 pagesImplant Details: Plan: Position: 33 SleeveRichard BalintNo ratings yet
- Stop Drill Kit - Ver.3Document16 pagesStop Drill Kit - Ver.3salehalsadiNo ratings yet
- TRUFIT CB PlugDocument4 pagesTRUFIT CB Plugapi-19808945No ratings yet
- Phoenix Urtks enDocument9 pagesPhoenix Urtks enJulek VargaNo ratings yet
- Risk Management Plan Form ExtraxtDocument4 pagesRisk Management Plan Form Extraxtpatricia colinNo ratings yet
- Neo Core Kit LeafletDocument2 pagesNeo Core Kit LeafletsalehalsadiNo ratings yet
- Side Element Um72 - 2959256Document4 pagesSide Element Um72 - 2959256RIYANo ratings yet
- Analytically Design and Tested The Flat Slab With Drop and Without Drop Under Punching Shear StrengthDocument8 pagesAnalytically Design and Tested The Flat Slab With Drop and Without Drop Under Punching Shear StrengthIJRASETPublicationsNo ratings yet
- Hip Prosthesis Design. Market Analysis, New Perspectives and An Innovative SolutionDocument5 pagesHip Prosthesis Design. Market Analysis, New Perspectives and An Innovative SolutionMohd BadrulNo ratings yet
- The Connection For Predictable BiologyDocument28 pagesThe Connection For Predictable BiologyRonaldoNo ratings yet
- LSGS-FOTB-O-48-DL DraftDocument7 pagesLSGS-FOTB-O-48-DL DraftTOTO HADIANINo ratings yet
- Handbook AgruDocument18 pagesHandbook AgruArnoldo OlivaNo ratings yet
- Catalog ConfidentDocument20 pagesCatalog ConfidentramukumarNo ratings yet
- EP19702124NWB1Document15 pagesEP19702124NWB1amanraza.canNo ratings yet
- Surgical Report: For ApprovalDocument7 pagesSurgical Report: For Approvalalosh60No ratings yet
- TDS587-17-P (PL6 BK) - SUMPRO New Standard WaDocument5 pagesTDS587-17-P (PL6 BK) - SUMPRO New Standard Waprismatama.kreasindoNo ratings yet
- Ultrasonic Testing Report: ResultsDocument1 pageUltrasonic Testing Report: ResultssamehNo ratings yet
- 2014 - Us8807241b2 - Devices and Method For Horizontal Directional Drilling With A Boring Tool LibraryDocument17 pages2014 - Us8807241b2 - Devices and Method For Horizontal Directional Drilling With A Boring Tool LibraryCường Nguyễn QuốcNo ratings yet
- WRIST-01030001 Distal Radius System 2.5 Step by Step PDFDocument25 pagesWRIST-01030001 Distal Radius System 2.5 Step by Step PDFUthman AlaoNo ratings yet
- Design and Computational Analysis of The Conventional Vaned Diffuser For A Turbocharger CompressorDocument8 pagesDesign and Computational Analysis of The Conventional Vaned Diffuser For A Turbocharger CompressorIJRASETPublicationsNo ratings yet
- ASD533 TD T140287en BDocument111 pagesASD533 TD T140287en Btame7478No ratings yet
- Accessories 1Document2 pagesAccessories 1femowox506No ratings yet
- GeM Bidding 5293644Document6 pagesGeM Bidding 5293644rakeshNo ratings yet
- 3M™ Half Facepiece Reusable Respirator 6200/07025 (AAD), Respiratory Protection, MediumDocument1 page3M™ Half Facepiece Reusable Respirator 6200/07025 (AAD), Respiratory Protection, MediumRichard Oswaldo Valenzuela MendietaNo ratings yet
- Piezoelectrico 2020 PDFDocument13 pagesPiezoelectrico 2020 PDFLucy Lobato RomeroNo ratings yet
- Eu Type-Examination Certificate Regulation (EU) 2016/425, MODULE B 0598/PPE/21/2588Document2 pagesEu Type-Examination Certificate Regulation (EU) 2016/425, MODULE B 0598/PPE/21/2588Amine ChlbNo ratings yet
- Application Guide Jotafloor Sealer: Thinner/Cleaning SolventDocument1 pageApplication Guide Jotafloor Sealer: Thinner/Cleaning SolventTamerTamerNo ratings yet
- ExpansorsDocument16 pagesExpansorssalahoveNo ratings yet
- Stokbord - InT-S 12mmDocument1 pageStokbord - InT-S 12mmMuhammad SyaifulhaqNo ratings yet
- FYTBK 30 TF Oval Flanged Ball Bearing Units - 20210917Document4 pagesFYTBK 30 TF Oval Flanged Ball Bearing Units - 20210917samNo ratings yet
- Implant SwissDocument19 pagesImplant SwissPhẩu PhanNo ratings yet
- US10130850Document16 pagesUS10130850Pardeep SharmaNo ratings yet
- Usd 795963Document11 pagesUsd 795963Víctor Manuel Martínez GarcíaNo ratings yet
- Us9729959 PDFDocument28 pagesUs9729959 PDFjohnmaxin1114No ratings yet
- Philos and Philos Long: Surgical TechniqueDocument38 pagesPhilos and Philos Long: Surgical TechniqueSamuel HarnaenNo ratings yet
- US8003261 AsahiDocument11 pagesUS8003261 AsahiSamshihNo ratings yet
- Design and Modification of Forming Tool in Press MachineDocument11 pagesDesign and Modification of Forming Tool in Press MachineIJRASETPublicationsNo ratings yet
- Defstan Compressed Air SystemsDocument166 pagesDefstan Compressed Air Systemsallah ditta shafiNo ratings yet
- Digital Imaging and Communications in Medicine (DICOM): A Practical Introduction and Survival GuideFrom EverandDigital Imaging and Communications in Medicine (DICOM): A Practical Introduction and Survival GuideNo ratings yet
- Thyroid Surgery DissertationDocument5 pagesThyroid Surgery DissertationDoMyPaperCanada100% (1)
- Minutes of Meeting On Perioperative NursingDocument5 pagesMinutes of Meeting On Perioperative NursingJeezreelNo ratings yet
- Regional Flaps in Head and Neck ReconstructionDocument11 pagesRegional Flaps in Head and Neck ReconstructionBenedetta GuarinoNo ratings yet
- Đề Thi Thử Lần 9.2019Document5 pagesĐề Thi Thử Lần 9.2019Hoa ĐỗNo ratings yet
- Evaluation of Cement-Retained Versus Screw-Retained Implant-Supported Restorations For Marginal Bone Loss A Systematic Review and Meta-AnalysisDocument9 pagesEvaluation of Cement-Retained Versus Screw-Retained Implant-Supported Restorations For Marginal Bone Loss A Systematic Review and Meta-AnalysisJuan Andres EspinozaNo ratings yet
- Urethral StrictureDocument35 pagesUrethral StrictureDoctors PodcastNo ratings yet
- Dinio Rad ContrastDocument1 pageDinio Rad ContrastLalaine De JesusNo ratings yet
- Visveswaraya Technological University: Belagavi - 590014, Karnataka, IndiaDocument11 pagesVisveswaraya Technological University: Belagavi - 590014, Karnataka, Indiarock starNo ratings yet
- Rehabilitation After Plate Fixation of Upper and Lower Extremity FracturesDocument6 pagesRehabilitation After Plate Fixation of Upper and Lower Extremity FractureswirasenaNo ratings yet
- Prevention and Management of Airway FireDocument19 pagesPrevention and Management of Airway Fireacegi135No ratings yet
- Summary:: Rodney B. SubaranDocument5 pagesSummary:: Rodney B. Subaranrodney subaranNo ratings yet
- Dissertation DentistryDocument4 pagesDissertation DentistryWriteMyPersuasivePaperSingapore100% (2)
- Post Mortem Care - RleDocument1 pagePost Mortem Care - RleXiamen Magsino NolascoNo ratings yet
- Female SterilizationDocument7 pagesFemale Sterilizationm90No ratings yet
- Section 4: Medical Suppliers Section 4: Medical Suppliers: Albert Massaad S.A.R.LDocument7 pagesSection 4: Medical Suppliers Section 4: Medical Suppliers: Albert Massaad S.A.R.LTannousNo ratings yet
- Mitral Valve Repair TrialDocument11 pagesMitral Valve Repair TrialAnawhatNo ratings yet
- Nabh Application Diagnostic Laboratories Imaging Centres PDFDocument35 pagesNabh Application Diagnostic Laboratories Imaging Centres PDFPrateek ChaharNo ratings yet
- Journal ClubDocument15 pagesJournal Clubperiodontics07No ratings yet
- Preprosthetic Surgery: An Adjunct To Complete Denture TherapyDocument3 pagesPreprosthetic Surgery: An Adjunct To Complete Denture TherapyrintanfsNo ratings yet
- Section 1: General Topics in Pediatric SurgeryDocument4 pagesSection 1: General Topics in Pediatric Surgeryகௌசிக் வெ0% (1)
- Midas Rex 8Document8 pagesMidas Rex 8Sidharth Kishore100% (1)
- The NeurocalometerDocument4 pagesThe NeurocalometernicoNo ratings yet
- I-Medi Rider & I-Medi Explus Rider: An Innovative Medical Plan That Protects You Now and Future Healthcare NeedsDocument39 pagesI-Medi Rider & I-Medi Explus Rider: An Innovative Medical Plan That Protects You Now and Future Healthcare NeedsDiyanah AkopNo ratings yet
- 2008-Australian Dental JournalDocument3 pages2008-Australian Dental Journaltea metaNo ratings yet
- Giudelines ERAS SC ERAS SocietyDocument8 pagesGiudelines ERAS SC ERAS SocietyNurul RezekiNo ratings yet
- Research ArticleDocument8 pagesResearch Articlekoas mr14No ratings yet