Professional Documents
Culture Documents
SurgicalReport Unapproved
Uploaded by
pyxk5c2yzmOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
SurgicalReport Unapproved
Uploaded by
pyxk5c2yzmCopyright:
Available Formats
Contact information:
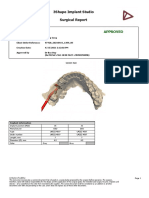
3Shape Implant Studio
Surgical Report
Order Details FOR APPROVAL
Patient Name:
Client Order Reference: 12345_20230916_2051_45_423
Creation Date: 9/16/2023 9:31:27 PM
Created by: Default user _______________
(121d801a-4663-4551-9172-fb9edc93371b) Approved by
Lower Jaw Upper Jaw
Implant information
Implant position (FDI) 25 27 45
Manufacturer Hiossen Hiossen Hiossen
Type ETIII Mini 3.5x10.0 ETIII Regular 4.0x8.5 ETIII Mini 3.5x10.0
Order number ET3M3510 ET3R4008 ET3M3510
Length, mm 10 8.5 10
Diameter (Ø), mm 3.5 4 3.5
Color Yellow Green Yellow
Implant information
Implant position (FDI) 47
Manufacturer Hiossen
Type ETIII Mini 3.5x8.5
Order number ET3M3508
Length, mm 8.5
Diameter (Ø), mm 3.5
Color Yellow
Limitation of Liability: Page 1
This instruction incorporates a custom document that is based on a surgical plan proposed by the surgeon before operation. The surgeon,
therefore, takes full medical responsibility for the design and the application of the surgical guide, the intended used surgical tray kit, implants and
sleeves – all as specified on the order form received by the supplier. The custom document shall be considered as an addition to all other
documents sent with and pertaining to the case, and it does not replace any of those other documents.
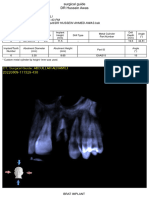
Implant Information
Implant position (FDI) 25
Manufacturer Hiossen
Type ETIII Mini 3.5x10.0
Order number ET3M3510
Length, mm 10
Diameter (Ø), mm 3.5
Color Yellow
Safety zone - apical distance 2.0
Safety zone - radial distance 1.5
Buccal Mesial Lingual Distal
M D
Limitation of Liability: Page 2
This instruction incorporates a custom document that is based on a surgical plan proposed by the surgeon before operation. The surgeon,
therefore, takes full medical responsibility for the design and the application of the surgical guide, the intended used surgical tray kit, implants and
sleeves – all as specified on the order form received by the supplier. The custom document shall be considered as an addition to all other
documents sent with and pertaining to the case, and it does not replace any of those other documents.
Implant Information
Implant position (FDI) 27
Manufacturer Hiossen
Type ETIII Regular 4.0x8.5
Order number ET3R4008
Length, mm 8.5
Diameter (Ø), mm 4
Color Green
Safety zone - apical distance 2.0
Safety zone - radial distance 1.5
Buccal Mesial Lingual Distal
M D
Limitation of Liability: Page 3
This instruction incorporates a custom document that is based on a surgical plan proposed by the surgeon before operation. The surgeon,
therefore, takes full medical responsibility for the design and the application of the surgical guide, the intended used surgical tray kit, implants and
sleeves – all as specified on the order form received by the supplier. The custom document shall be considered as an addition to all other
documents sent with and pertaining to the case, and it does not replace any of those other documents.
Implant Information
Implant position (FDI) 45
Manufacturer Hiossen
Type ETIII Mini 3.5x10.0
Order number ET3M3510
Length, mm 10
Diameter (Ø), mm 3.5
Color Yellow
Safety zone - apical distance 2.0
Safety zone - radial distance 1.5
Buccal Mesial Lingual Distal
M D
Limitation of Liability: Page 4
This instruction incorporates a custom document that is based on a surgical plan proposed by the surgeon before operation. The surgeon,
therefore, takes full medical responsibility for the design and the application of the surgical guide, the intended used surgical tray kit, implants and
sleeves – all as specified on the order form received by the supplier. The custom document shall be considered as an addition to all other
documents sent with and pertaining to the case, and it does not replace any of those other documents.
Implant Information
Implant position (FDI) 47
Manufacturer Hiossen
Type ETIII Mini 3.5x8.5
Order number ET3M3508
Length, mm 8.5
Diameter (Ø), mm 3.5
Color Yellow
Safety zone - apical distance 2.0
Safety zone - radial distance 1.5
Buccal Mesial Lingual Distal
M D
Limitation of Liability: Page 5
This instruction incorporates a custom document that is based on a surgical plan proposed by the surgeon before operation. The surgeon,
therefore, takes full medical responsibility for the design and the application of the surgical guide, the intended used surgical tray kit, implants and
sleeves – all as specified on the order form received by the supplier. The custom document shall be considered as an addition to all other
documents sent with and pertaining to the case, and it does not replace any of those other documents.
You might also like
- Short ImplantsFrom EverandShort ImplantsBoyd J. TomasettiNo ratings yet
- 3shape Implant Studio Surgical Report: ApprovedDocument3 pages3shape Implant Studio Surgical Report: Approvednha khoa NHƯ NGỌCNo ratings yet
- 3shape Implant Studio Drilling ProtocolDocument2 pages3shape Implant Studio Drilling Protocolnha khoa NHƯ NGỌCNo ratings yet
- 3shape Implant Studio Surgical Report: ApprovedDocument3 pages3shape Implant Studio Surgical Report: Approvednha khoa NHƯ NGỌCNo ratings yet
- Surgical Report: For ApprovalDocument7 pagesSurgical Report: For Approvalalosh60No ratings yet
- 3shape Implant Studio Drilling Protocol: Contact InformationDocument2 pages3shape Implant Studio Drilling Protocol: Contact Informationnha khoa NHƯ NGỌCNo ratings yet
- Surgical ReportDocument8 pagesSurgical ReportTiago Oliveira ImperiortoNo ratings yet
- Drilling ProtocolDocument1 pageDrilling ProtocolEdwin Lupaca ArocutipaNo ratings yet
- Implant Details: Plan: Position: 33 SleeveDocument2 pagesImplant Details: Plan: Position: 33 SleeveRichard BalintNo ratings yet
- Exocad Surgical Plan ModelDocument2 pagesExocad Surgical Plan ModelFernando Pinto AlvarezNo ratings yet
- NomogramDocument1 pageNomogramSPOSI MTAurangabadNo ratings yet
- TRUFIT CB PlugDocument4 pagesTRUFIT CB Plugapi-19808945No ratings yet
- Implant Materials, Designs, and Surfaces 2021Document18 pagesImplant Materials, Designs, and Surfaces 2021Mohamed GabrNo ratings yet
- ExpansorsDocument16 pagesExpansorssalahoveNo ratings yet
- 09 Nail-Instruction For UseDocument4 pages09 Nail-Instruction For UseDiana Rodriguez ParodiNo ratings yet
- The Connection For Predictable BiologyDocument28 pagesThe Connection For Predictable BiologyRonaldoNo ratings yet
- Omny Classics Catalogue and Manual - enDocument52 pagesOmny Classics Catalogue and Manual - enIsi-journals PublisherNo ratings yet
- 20030e Trenton Corp Patchpad Rev7Document2 pages20030e Trenton Corp Patchpad Rev7Rohammed CastilloNo ratings yet
- Catalog ConfidentDocument20 pagesCatalog ConfidentramukumarNo ratings yet
- Surgical GuideDocument29 pagesSurgical GuideVăn Trọng MinhNo ratings yet
- TNex Sman 7-8Document7 pagesTNex Sman 7-8chauphenNo ratings yet
- PIIS0022391322004668Document9 pagesPIIS0022391322004668LalaNo ratings yet
- ETIII Ø3.2 Implant: Conventional Implant For Restrictive SpacesDocument2 pagesETIII Ø3.2 Implant: Conventional Implant For Restrictive SpacesImplant DentNo ratings yet
- Ridge Wider Kit Leaflet - 20.01.13Document2 pagesRidge Wider Kit Leaflet - 20.01.13salehalsadiNo ratings yet
- GeM Bidding 5293644Document6 pagesGeM Bidding 5293644rakeshNo ratings yet
- An Interocclusal Recording Method For The Fabrication of Full-Arch Implant-Retained RestorationsDocument9 pagesAn Interocclusal Recording Method For The Fabrication of Full-Arch Implant-Retained RestorationsrayaimNo ratings yet
- Shielding Design Methods For Radiation Oncology DepartmentsDocument206 pagesShielding Design Methods For Radiation Oncology DepartmentsAhmed AwshanNo ratings yet
- A - FLYER MicroMega One RECI EN - WEBDocument2 pagesA - FLYER MicroMega One RECI EN - WEBHugo Alonso Porras AlvarezNo ratings yet
- Stop Drill Kit - Ver.3Document16 pagesStop Drill Kit - Ver.3salehalsadiNo ratings yet
- Manual Vision Elect 26Document29 pagesManual Vision Elect 26ismael100% (1)
- An Interocclusal Recording Method For The Fabrication of Full-Arch Implant-Retained RestorationsDocument9 pagesAn Interocclusal Recording Method For The Fabrication of Full-Arch Implant-Retained RestorationsDr FarhatNo ratings yet
- Hoja de Medición y Planeación TAA (Isolated)Document2 pagesHoja de Medición y Planeación TAA (Isolated)Fco CasNo ratings yet
- Zeiss Ant Seg Sale Sheet CIR11038Document2 pagesZeiss Ant Seg Sale Sheet CIR11038Raúl Plasencia SaliniNo ratings yet
- Tooth Structure Removal Associated With Various Preparation Designs For Anterior TeethDocument7 pagesTooth Structure Removal Associated With Various Preparation Designs For Anterior TeethAlina AnechiteiNo ratings yet
- Product CatalogDocument92 pagesProduct CatalogdoctorniravNo ratings yet
- Biotec Dental Implants Conical Connection B1 Catalogue 2019Document8 pagesBiotec Dental Implants Conical Connection B1 Catalogue 2019Biotec implant systems GmbHNo ratings yet
- Expander System Dimension Sheet - Stepped Pin Type CDocument2 pagesExpander System Dimension Sheet - Stepped Pin Type CebraNo ratings yet
- Syringe Test Fixture For ISO 7886 1Document2 pagesSyringe Test Fixture For ISO 7886 1Samrat SinghaNo ratings yet
- PB Nuflow REV03Document2 pagesPB Nuflow REV03metech8xNo ratings yet
- GL - PROC - PRO-S - IMAGINA - Scopes - Fact Sheet - 01.2020Document2 pagesGL - PROC - PRO-S - IMAGINA - Scopes - Fact Sheet - 01.2020rq yNo ratings yet
- AB Catalogue 2013Document150 pagesAB Catalogue 2013AhmadModiNo ratings yet
- Insulating Kit: SpecificationDocument1 pageInsulating Kit: SpecificationNawazNo ratings yet
- Ejector MultiTool - WZ73enDocument12 pagesEjector MultiTool - WZ73enBebe Ionuț AnițaNo ratings yet
- PB Miniflow Long REV01Document3 pagesPB Miniflow Long REV01AZIZ UR RAHMANNo ratings yet
- 2007 JomiDocument7 pages2007 JomiCássio BernardNo ratings yet
- GuideMia Sample Report - EnglishDocument9 pagesGuideMia Sample Report - Englishzhiao liuNo ratings yet
- Etu Ortho Surgical Parts PDFDocument18 pagesEtu Ortho Surgical Parts PDFShivNo ratings yet
- Narrow Diameter Dental Implant: Reporter ModeratorDocument71 pagesNarrow Diameter Dental Implant: Reporter ModeratorkangleisNo ratings yet
- Facial ContoursDocument20 pagesFacial Contourscmkflorida7011100% (1)
- One System. Several Custom Configurations.: Zimmer Instrument Kit SystemDocument3 pagesOne System. Several Custom Configurations.: Zimmer Instrument Kit Systemjitendertalwar1603No ratings yet
- DFM Guidebook Sheetmetal Design Guidelines Issue XVDocument11 pagesDFM Guidebook Sheetmetal Design Guidelines Issue XVRushil ShahNo ratings yet
- Risk Management Plan Form ExtraxtDocument4 pagesRisk Management Plan Form Extraxtpatricia colinNo ratings yet
- Visible Adaptability: DERIVO® Embolisation DeviceDocument8 pagesVisible Adaptability: DERIVO® Embolisation DeviceJose Gregorio Rosales MenesesNo ratings yet
- Za Removable Attachment L8024 Locator RTX Manual TMDocument24 pagesZa Removable Attachment L8024 Locator RTX Manual TMbarrahamzawiNo ratings yet
- Catalogo Lista Codici Protesi-Fissa-Ot-Bridge 2022 ENG MOD D375SP Rev 08 Del 11-02-2022 VERSIONE WEB CompressedDocument20 pagesCatalogo Lista Codici Protesi-Fissa-Ot-Bridge 2022 ENG MOD D375SP Rev 08 Del 11-02-2022 VERSIONE WEB CompressedThanh Nhan DinhNo ratings yet
- Eg 202670v010102pDocument98 pagesEg 202670v010102phadiranjiNo ratings yet
- NL Trigen Meta Nail Retrograde SurgicaltechniqueDocument68 pagesNL Trigen Meta Nail Retrograde SurgicaltechniqueSara MorenoNo ratings yet
- Straumann® Guided Surgery System InstrumentsDocument48 pagesStraumann® Guided Surgery System InstrumentsSupaluk Mod ChuencheepNo ratings yet
- Philos and Philos Long: Surgical TechniqueDocument38 pagesPhilos and Philos Long: Surgical TechniqueSamuel HarnaenNo ratings yet
- No21 System INVISIO Vertical FacadesDocument24 pagesNo21 System INVISIO Vertical FacadesKitanovic NenadNo ratings yet
- Fixed Functional AppliancesDocument82 pagesFixed Functional Appliancesdisha 146jandialNo ratings yet
- 02-Three-Visit CD PDFDocument20 pages02-Three-Visit CD PDFBernythefly axcNo ratings yet
- FacemaskDocument30 pagesFacemaskAnushree RathiNo ratings yet
- Dental Education in ThailandDocument5 pagesDental Education in ThailandDent YomarajNo ratings yet
- Unit 4 Management of Open Pulp in Deciduous TeethDocument11 pagesUnit 4 Management of Open Pulp in Deciduous Teethisti DaristiviaNo ratings yet
- Tiologia Class 3Document24 pagesTiologia Class 3Joyce GalvezNo ratings yet
- Restorative Materials (Dental Composite)Document60 pagesRestorative Materials (Dental Composite)فواز نميرNo ratings yet
- Oral Assessment and Care PDFDocument3 pagesOral Assessment and Care PDFAbdallah AlwawiNo ratings yet
- Entry 3 Reading Sample Paper 1 CA v3 Dec17Document10 pagesEntry 3 Reading Sample Paper 1 CA v3 Dec17lilalilak0% (1)
- Habit Breaking Appliance For Tongue Thrusting - A ModificationDocument5 pagesHabit Breaking Appliance For Tongue Thrusting - A ModificationsrinandanNo ratings yet
- Introduction To Pediatric DentistryDocument23 pagesIntroduction To Pediatric Dentistrydr parveen bathla0% (1)
- 3D Technologies For Precision in Orthodontics - 2018 - Seminars in Orthodontics PDFDocument7 pages3D Technologies For Precision in Orthodontics - 2018 - Seminars in Orthodontics PDFOmy J. CruzNo ratings yet
- Fully Digital Workflow, Integrating Dental Scan, Smile Design and CAD-CAM: Case ReportDocument13 pagesFully Digital Workflow, Integrating Dental Scan, Smile Design and CAD-CAM: Case ReportCarlosChavezRiosNo ratings yet
- Role of CBCT in Implant Placement in Mandibular Premolar RegionDocument6 pagesRole of CBCT in Implant Placement in Mandibular Premolar RegionIJAR JOURNALNo ratings yet
- Why Is History Taking Important? Dental or Medical: Gingival HyperplasiaDocument20 pagesWhy Is History Taking Important? Dental or Medical: Gingival HyperplasiaFe RiveraNo ratings yet
- Eja 190160mkDocument6 pagesEja 190160mkOsama GamilNo ratings yet
- Consensus On The Avoidance and Management of Complications of Implant Based Treatment Foundation For Oral Rehabilitation - RevDocument180 pagesConsensus On The Avoidance and Management of Complications of Implant Based Treatment Foundation For Oral Rehabilitation - RevCamila Bórquez SchenckeNo ratings yet
- Article Fluoride WaterDocument3 pagesArticle Fluoride Waterjust achickNo ratings yet
- Analysis of C-Shaped Canals by Panoramic Radiography and Cone-Beam Computed Tomography - Root-Type Specificity by Longitudinal DistributionDocument5 pagesAnalysis of C-Shaped Canals by Panoramic Radiography and Cone-Beam Computed Tomography - Root-Type Specificity by Longitudinal DistributionAnthony LiNo ratings yet
- Efficient Esthetics With Neo Spectra™ ST CompositesDocument4 pagesEfficient Esthetics With Neo Spectra™ ST CompositesWillianNo ratings yet
- Lec-14-15-Technical-Steps-Compressed Finishing Polishing MetalframeDocument15 pagesLec-14-15-Technical-Steps-Compressed Finishing Polishing MetalframeSalsa NPSNo ratings yet
- Dental Drill: HistoryDocument4 pagesDental Drill: HistoryFatih DemirNo ratings yet
- Darkie (CC)Document13 pagesDarkie (CC)vaanangNo ratings yet
- 1 SMDocument7 pages1 SMGhina AdilahNo ratings yet
- NFSUDocument14 pagesNFSUROMESHA CHATTERJEENo ratings yet
- GuideMia Sample Report - EnglishDocument9 pagesGuideMia Sample Report - Englishzhiao liuNo ratings yet
- Dental Catalog-2019-WebDocument74 pagesDental Catalog-2019-WebKothapalli ChiranjeeviNo ratings yet
- Lec. Deep CariesDocument47 pagesLec. Deep CariesMaria EvergardenNo ratings yet
- Practice MCQs of Block 4, 5 & 6 - Consider ThisDocument53 pagesPractice MCQs of Block 4, 5 & 6 - Consider ThisIkram UddinNo ratings yet
- Treatment of Midline DiastemaDocument4 pagesTreatment of Midline Diastemamohamed gamalNo ratings yet