Professional Documents
Culture Documents
3shape Implant Studio Drilling Protocol: Contact Information
Uploaded by
nha khoa NHƯ NGỌCOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
3shape Implant Studio Drilling Protocol: Contact Information
Uploaded by
nha khoa NHƯ NGỌCCopyright:
Available Formats
Contact information:
3Shape Implant
Studio
Drilling Protocol
Order Details
Patient Name: Tuong Cong
Client Order Reference: 97728_20210415_1359_05
Creation Date: 4/15/2021 2:08:44 PM
Created by: Dr Bao Duy
(0e7819ef-c561-4b98-9627-c7bf8025089b)
*1=Note: Please check the required final drill length against the suggested minimum drill length for your chosen implants. The final drill length depends on the chosen sleeve type and instructions for use for
the relevant surgical protocol defined by your implant choice. E.g., for fully guided sleeves additional values might need to be added like a potentially used spoon or extended drill depth.
Implant information
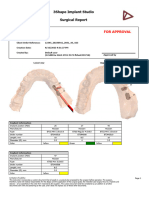
Implant position (FDI) 35
Manufacturer DIO
Type UF(II) 4507
Order number UF(II) 4507
Length, mm 7
Diameter (Ø), mm 4.5
Color Gray
Sleeve information
Name DIO GS 53
Type Fully guided
Order number GS 53
Offset, mm 9
Color Blue
Drill information
Minimum drill length 16
Limitation of Liability: Page 1
This instruction incorporates a custom document that is based on a surgical plan proposed by the surgeon before operation. The surgeon, therefore, takes full medical responsibility for
the design and the application of the surgical guide, the intended used surgical tray kit, implants and sleeves – all as specified on the order form received by the supplier. The custom
document shall be considered as an addition to all other documents sent with and pertaining to the case, and it does not replace any of those other documents.
Implant information
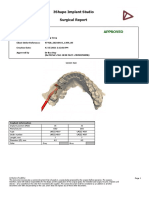
Implant position (FDI) 36
Manufacturer DIO
Type UF(II) 4507
Order number UF(II) 4507
Length, mm 7
Diameter (Ø), mm 4.5
Color Gray
Sleeve information
Name DIO GS 53
Type Fully guided
Order number GS 53
Offset, mm 9
Color Blue
Drill information
Minimum drill length 16
Limitation of Liability: Page 2
This instruction incorporates a custom document that is based on a surgical plan proposed by the surgeon before operation. The surgeon, therefore, takes full medical responsibility for
the design and the application of the surgical guide, the intended used surgical tray kit, implants and sleeves – all as specified on the order form received by the supplier. The custom
document shall be considered as an addition to all other documents sent with and pertaining to the case, and it does not replace any of those other documents.
You might also like
- RISK MANAGEMENT Report ExampleDocument4 pagesRISK MANAGEMENT Report Examplepatricia colin100% (1)
- Neodent Guided Kit PDFDocument24 pagesNeodent Guided Kit PDFBehdad JavdanNo ratings yet
- Checklist For 3D Model ReviewDocument9 pagesChecklist For 3D Model ReviewMONER MANUS100% (1)
- Introduction To Process Plant Start-Up and CommissioningDocument35 pagesIntroduction To Process Plant Start-Up and Commissioning2091979100% (6)
- Minimally Invasive Dental Implant SurgeryFrom EverandMinimally Invasive Dental Implant SurgeryDaniel R. CullumNo ratings yet
- Fall Management Technical File DoC EU MDRDocument3 pagesFall Management Technical File DoC EU MDRvicNo ratings yet
- LC - Gunk SqueezesDocument5 pagesLC - Gunk SqueezesHeris SitompulNo ratings yet
- Title of Thesis or Practicum or Research Proposal or Report: Firstname M. LastnameDocument17 pagesTitle of Thesis or Practicum or Research Proposal or Report: Firstname M. LastnameDozdi100% (3)
- Lump Sum Contract NotesDocument1 pageLump Sum Contract NotesamrkiplNo ratings yet
- US20190376758A1Document8 pagesUS20190376758A1Jean DelaronciereNo ratings yet
- BFD-180-570 DN65 GBDocument25 pagesBFD-180-570 DN65 GBalexander100% (3)
- Protaper TechniqueDocument14 pagesProtaper TechniquePiyush Varshney100% (1)
- Seam 325 Prelim Exam ReviewerDocument6 pagesSeam 325 Prelim Exam ReviewerKyle Steven CaymeNo ratings yet
- Autonomy of Art or Dignity of The Artwork? - Agnes HellerDocument20 pagesAutonomy of Art or Dignity of The Artwork? - Agnes HellerProfrFer100% (1)
- Exocad Surgical Plan ModelDocument2 pagesExocad Surgical Plan ModelFernando Pinto AlvarezNo ratings yet
- Angeles, Rea P - Unit 1 - Methods in Teaching Industrial ArtsDocument2 pagesAngeles, Rea P - Unit 1 - Methods in Teaching Industrial ArtsEthel Rose SorianoNo ratings yet
- 3shape Implant Studio Drilling ProtocolDocument2 pages3shape Implant Studio Drilling Protocolnha khoa NHƯ NGỌCNo ratings yet
- Drilling ProtocolDocument1 pageDrilling ProtocolEdwin Lupaca ArocutipaNo ratings yet
- SurgicalReport UnapprovedDocument5 pagesSurgicalReport Unapprovedpyxk5c2yzmNo ratings yet
- Surgical ReportDocument8 pagesSurgical ReportTiago Oliveira ImperiortoNo ratings yet
- 3shape Implant Studio Surgical Report: ApprovedDocument3 pages3shape Implant Studio Surgical Report: Approvednha khoa NHƯ NGỌCNo ratings yet
- 3shape Implant Studio Surgical Report: ApprovedDocument3 pages3shape Implant Studio Surgical Report: Approvednha khoa NHƯ NGỌCNo ratings yet
- TRUFIT CB PlugDocument4 pagesTRUFIT CB Plugapi-19808945No ratings yet
- NomogramDocument1 pageNomogramSPOSI MTAurangabadNo ratings yet
- 09 Nail-Instruction For UseDocument4 pages09 Nail-Instruction For UseDiana Rodriguez ParodiNo ratings yet
- Multipoint Rod Extensometer: User ManualDocument20 pagesMultipoint Rod Extensometer: User ManualAndres MejiaNo ratings yet
- Neo Core Kit LeafletDocument2 pagesNeo Core Kit LeafletsalehalsadiNo ratings yet
- US10130850Document16 pagesUS10130850Pardeep SharmaNo ratings yet
- Ultrasonic Testing Report: ResultsDocument1 pageUltrasonic Testing Report: ResultssamehNo ratings yet
- End Cover - D-PTTB 4-TG - 3211918: Key Commercial DataDocument3 pagesEnd Cover - D-PTTB 4-TG - 3211918: Key Commercial DataArvin PNo ratings yet
- Analytically Design and Tested The Flat Slab With Drop and Without Drop Under Punching Shear StrengthDocument8 pagesAnalytically Design and Tested The Flat Slab With Drop and Without Drop Under Punching Shear StrengthIJRASETPublicationsNo ratings yet
- Dental Card-E Rev.09-13 - EnGDocument24 pagesDental Card-E Rev.09-13 - EnGTabure22No ratings yet
- Surgical GuideDocument29 pagesSurgical GuideVăn Trọng MinhNo ratings yet
- MIS C1 CatalogDocument36 pagesMIS C1 CatalogJorge SaenzNo ratings yet
- Stop Drill Kit - Ver.3Document16 pagesStop Drill Kit - Ver.3salehalsadiNo ratings yet
- Catalog ConfidentDocument20 pagesCatalog ConfidentramukumarNo ratings yet
- Risk Management Plan Form ExtraxtDocument4 pagesRisk Management Plan Form Extraxtpatricia colinNo ratings yet
- Surgical Report: For ApprovalDocument7 pagesSurgical Report: For Approvalalosh60No ratings yet
- Implant Details: Plan: Position: 33 SleeveDocument2 pagesImplant Details: Plan: Position: 33 SleeveRichard BalintNo ratings yet
- Piezoelectrico 2020 PDFDocument13 pagesPiezoelectrico 2020 PDFLucy Lobato RomeroNo ratings yet
- United States Design Patent (10) Patent No .:: (45) Date of Patent: May 7, 2019Document6 pagesUnited States Design Patent (10) Patent No .:: (45) Date of Patent: May 7, 2019NadineNo ratings yet
- Implant SwissDocument19 pagesImplant SwissPhẩu PhanNo ratings yet
- Stokbord - InT-S 12mmDocument1 pageStokbord - InT-S 12mmMuhammad SyaifulhaqNo ratings yet
- The Connection For Predictable BiologyDocument28 pagesThe Connection For Predictable BiologyRonaldoNo ratings yet
- Philos and Philos Long: Surgical TechniqueDocument38 pagesPhilos and Philos Long: Surgical TechniqueSamuel HarnaenNo ratings yet
- Ppiezosurgery in Third Molars A Review of Literaturep PDFDocument5 pagesPpiezosurgery in Third Molars A Review of Literaturep PDFdeninikeNo ratings yet
- Handbook AgruDocument18 pagesHandbook AgruArnoldo OlivaNo ratings yet
- WRIST-01030001 Distal Radius System 2.5 Step by Step PDFDocument25 pagesWRIST-01030001 Distal Radius System 2.5 Step by Step PDFUthman AlaoNo ratings yet
- ASD533 TD T140287en BDocument111 pagesASD533 TD T140287en Btame7478No ratings yet
- US8611813Document19 pagesUS8611813ahpatentservicesNo ratings yet
- Dep 22 1680 QD GDR217BS4371 - 20231212105031.905 - XDocument10 pagesDep 22 1680 QD GDR217BS4371 - 20231212105031.905 - XoswaldovbNo ratings yet
- EP19702124NWB1Document15 pagesEP19702124NWB1amanraza.canNo ratings yet
- 34054ft-Bata TNT Blanca CcintaDocument2 pages34054ft-Bata TNT Blanca CcintaPepa GarcíaNo ratings yet
- 3M™ Half Facepiece Reusable Respirator 6200/07025 (AAD), Respiratory Protection, MediumDocument1 page3M™ Half Facepiece Reusable Respirator 6200/07025 (AAD), Respiratory Protection, MediumRichard Oswaldo Valenzuela MendietaNo ratings yet
- Us9729959 PDFDocument28 pagesUs9729959 PDFjohnmaxin1114No ratings yet
- 2014 - Us8807241b2 - Devices and Method For Horizontal Directional Drilling With A Boring Tool LibraryDocument17 pages2014 - Us8807241b2 - Devices and Method For Horizontal Directional Drilling With A Boring Tool LibraryCường Nguyễn QuốcNo ratings yet
- Phoenix Urtks enDocument9 pagesPhoenix Urtks enJulek VargaNo ratings yet
- Side Element Um72 - 2959256Document4 pagesSide Element Um72 - 2959256RIYANo ratings yet
- United States Patent: Cheng Et Al. (10) Patent No.: US 9,078,547 B2Document10 pagesUnited States Patent: Cheng Et Al. (10) Patent No.: US 9,078,547 B2Alex BrcNo ratings yet
- Usd 795963Document11 pagesUsd 795963Víctor Manuel Martínez GarcíaNo ratings yet
- One Shape: One Single File in Continuous RotationDocument24 pagesOne Shape: One Single File in Continuous RotationJL' CardosoNo ratings yet
- United States Patent (10) Patent No.: US 9.222,183 B2Document16 pagesUnited States Patent (10) Patent No.: US 9.222,183 B2turnipNo ratings yet
- PatentDocument8 pagesPatentRikyNo ratings yet
- Defstan Compressed Air SystemsDocument166 pagesDefstan Compressed Air Systemsallah ditta shafiNo ratings yet
- U.S. Pat. 9,760,385-Concurrent Emulation of Multiple Devices-2013 (Emulator) PDFDocument75 pagesU.S. Pat. 9,760,385-Concurrent Emulation of Multiple Devices-2013 (Emulator) PDFDuane BlakeNo ratings yet
- Whitley Bencharit Formlabs Whitepaper2016Document15 pagesWhitley Bencharit Formlabs Whitepaper2016SadishNo ratings yet
- Us9976567 (B2)Document10 pagesUs9976567 (B2)Gabriel PhilippiNo ratings yet
- Graftless Solutions for the Edentulous PatientFrom EverandGraftless Solutions for the Edentulous PatientSaj JivrajNo ratings yet
- Electronic Unit Injector - Remove: C6.6 Industrial EngineDocument12 pagesElectronic Unit Injector - Remove: C6.6 Industrial EngineBassieNo ratings yet
- Development LetterDocument3 pagesDevelopment Lettertan balanNo ratings yet
- CGC TemplateDocument19 pagesCGC TemplateVictoria Stephanie AshleyNo ratings yet
- Publishing Misconduct: A Need of Understanding and ReductionDocument7 pagesPublishing Misconduct: A Need of Understanding and ReductionLavanyaNo ratings yet
- Cat-Eht Brake New PDFDocument4 pagesCat-Eht Brake New PDFLingaraj Suresh LingaianNo ratings yet
- Telecom - MA - Update 2019 - vFINALDocument11 pagesTelecom - MA - Update 2019 - vFINALYandhi SuryaNo ratings yet
- B.tech 3 Cse SyllabusDocument65 pagesB.tech 3 Cse SyllabusVijayendra GoudNo ratings yet
- Vis DK 25 Programming GuideDocument210 pagesVis DK 25 Programming GuideshrihnNo ratings yet
- GP-100 Software User Guide This Software Only Supports Windows SystemDocument1 pageGP-100 Software User Guide This Software Only Supports Windows SystemJohn HenryNo ratings yet
- LFS EksellDisplayDocument7 pagesLFS EksellDisplayillustratio.academyNo ratings yet
- F2-14 Budget PreparationDocument18 pagesF2-14 Budget PreparationJaved Imran100% (1)
- PI Performance Expectations CAT 2022Document46 pagesPI Performance Expectations CAT 2022Gursanjan TiwanaNo ratings yet
- Module 5 Advanced MechanicsDocument60 pagesModule 5 Advanced Mechanicsiknowvictoriassecret49No ratings yet
- Reading Comprehension Read The Article Below and Then Answer The Questions That FollowDocument4 pagesReading Comprehension Read The Article Below and Then Answer The Questions That Followjuan m isazaNo ratings yet
- 3.1 Mitosis Ans PDFDocument7 pages3.1 Mitosis Ans PDFtess_15No ratings yet
- MLAHomeworkDocument4 pagesMLAHomeworkVANESSA SALDANANo ratings yet
- BMI Middle East and Africa Oil and Gas Insight March 2016Document10 pagesBMI Middle East and Africa Oil and Gas Insight March 2016Anonymous pPpTQrpvbrNo ratings yet
- Manual Safety SignDocument49 pagesManual Safety SignMuamar DhikriNo ratings yet
- Aileen Flores Lesson Plan 2021Document8 pagesAileen Flores Lesson Plan 2021Sissay SemblanteNo ratings yet
- GSE580Document132 pagesGSE580Anonymous g4wR41qNeNo ratings yet
- Data Archiving in Enterprise Controlling (EC)Document16 pagesData Archiving in Enterprise Controlling (EC)sf69vNo ratings yet
- Piyush Mulkar: Cleveland, OHDocument6 pagesPiyush Mulkar: Cleveland, OHRadha RamineniNo ratings yet