Professional Documents
Culture Documents
GGT Activity Test
Uploaded by
محمد رحيم حسن محمودOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
GGT Activity Test
Uploaded by
محمد رحيم حسن محمودCopyright:
Available Formats
BIOLABO
www.biolabo.fr
GAMMA-GT carboxy GPNA
MANUFACTURER: Reagent for quantitative determination of
BIOLABO SAS,
Gamma Glutamyltransferase activity [ EC 2.3.2.2 ] in human serum and plasma.
Les Hautes Rives
02160, Maizy, France
REF 81110 R1 8 x 30 mL R2 8 x 30 mL
REF 81210 R1 1 x 105 mL R2 10 x 10 mL
REF 81310 R1 10 x 100 mL R2 10 x 100 mL
|
TECHNICAL SUPPORT AND ORDERS
Tel: (33) 03 23 25 15 50
IVD IN VITRO DIAGNOSTIC USE
Fax: (33) 03 23 256 256
CLINICAL SIGNIFICANCE (1) (2) REAGENT PREPARATION
Even though renal tissue has the highest level of γ-glutamyltransferase Use a non-sharp instrument to remove aluminium cap.
(GGT), the enzyme present in serum appears to originate primarily REF 81210: Add promptly 10 mL of vial R1 (Buffer) into the contents
from the hepatobiliary system. GGT activity is elevated in any and all of vial R2 (Substrate).
forms of liver disease. It is highest in cases of intrahepatic or
posthepatic biliary obstruction. It is more sensitive than ALP and Others REF: Dilute the contents of vial R2 (Substrate) with approx.
transaminases in detecting obstructive jaundice, cholangitis and 10 mL of vial R1 (Buffer), transfer into vial R1 (buffer).
cholecystitis. Only moderate elevation occurs in infectious hepatitis, Mix gently and wait for complete dissolution before using reagent
chronic alcoholism or drugs use (sedatives, anticonvulsants,and (approximately 2 minutes).
tranquilisers). Decreased GGT activity is found in case of
hypothyroidism.
In summary, GGT is the most sensitive enzymatic indicator of STABILITY AND STORAGE
hepatobiliary disease available at present but doesn’t allow Store away from light, well cap in the original vial at 2-8°C
discriminating between different kinds of liver disease. • Unopened, reagents are stable until expiry date stated on the label
of the kit when stored and used as described in the insert.
PRINCIPLE (4) (5) • Once reconstituted, working reagent is stable for 30 days when free
Szasz, Rosalki and Tarlow method. Reaction scheme is as follows: from contamination.
• Discard any reagent if cloudy or if reagent blank at 405 nm > 1.000
L-G-Glutamyl-3-carboxy-4- GGT L-G-Glutamyl-glycylglycine
nitroanilide • Don’t use working reagent after expiry date stated on the label of the
+ p-nitroaniline
+ Glyc ylglycine Kit.
The rate of formation of p-nitroaniline, directly proportional to SPECIMEN COLLECTION AND HANDLING (1) (2)
GGT activity in the specimen, is measured at 405 nm. Unhemolysed serum or EDTA-plasma (up to 1 mg/ml of blood).
Heparin produces turbidity in the reaction mixture; citrate, oxalate and
fluoride depress GGT activity by 10 to 15%.
REAGENTS COMPOSITION
GGT is stable in serum for:
Vial R1 BUFFER • 1 month at 2-8°C
• 1 year at–20°C.
Glycylglycine 100 mmol/L
TRIS pH 8.25 95 mmol/L
Preservative INTERFERENCES (1) (3)
Tests results on Kenza 240 TX:
Vial R2 SUBSTRATE Ascorbic Acid: No interference up to 25 mg/dL.
Total Bilirubin: Negative interference over 450 µmol/L.
L-G-glutamyl-3-carboxy-4-nitroanilide 80 mmol/L Glucose: No interference.
(Carboxy-GPNA) Haemolysis: Negative interference over 225 µmol/L.
Turbidity: No interference.
For a more comprehensive review of factors affecting this assay refer to
SAFETY CAUTIONS the publication of Young D.S.
BIOLABO reagents are designated for professional, in vitro diagnostic
use.
MATERIALS REQUIRED BUT NOT PROVIDED
• Verify the integrity of the contents before use.
• Nitroaniline derivatives (product of the reaction) are toxic. Do not 1. Medical analysis laboratory equipment.
inhale, do not ingest. 2. Normal and pathological control sera.
• Use adequate protections (overall, gloves, glasses).
• Do not pipette by mouth.
• In case of contact with skin or eyes, thoroughly wash affected areas CALIBRATION
with plenty of water and seek medical advice. Results will depend on the accuracy of the instrument calibration, the
• Reagents contain sodium azide (concentration < 0,1%) which may time counting, the respect of reagent/specimen ratio and the
react with copper and lead plumbing. Flush with plenty of water temperature control.
when disposing. • Use the theoretical calibration factor (§ CALCULATION)
• Material Safety Data Sheet is available upon request. • Or REF 95015 BIOLABO Multicalibrator (calibration value
• Waste disposal: Respect legislation in force in the country. determined with validated statistical techniques and metrologically
All specimens should be handled as potentially infectious, in controlled instrument)
accordance with good laboratory practices using appropriate • Or a multiparametric calibrator traceable to a reference method or
precautions. Respect legislation in force in the country. material.
Made in France Latest revision : www.biolabo.fr Revision : 19/06/2013
QUALITY CONTROL MANUAL PROCEDURE
• BIOLABO EXATROL-N Level I REF 95010 Let stand reagents and specimens at room temperature.
• BIOLABO EXATROL-P Level II REF 95011
• Other assayed control sera referring to the same method. Pipette into 1 cm path length thermostated cuvette:
• External quality control program.
It is recommended to control in the following cases: Reagent 1 mL
• At least once a run. Bring to 37°C (30°C) then add:
• At least once within 24 hours.
• When changing vial of reagent. Specimen or Calibrator 50 µL
• After maintenance operations on the instrument.
If control is out of range, apply following actions: Mix. Read initial absorbance after 30 seconds, record absorbance at 405 nm
1. Repeat the test with the same control. every minute during 3 minutes.
2. If control is still out of range, prepare a fresh control serum and Calculate absorbance change per minute (∆Abs/min).
repeat the test.
3. If control is still out of range, verify analysis parameters: Notes: Specific procedures are available upon request for
Wavelength, temperature, specimen/reagent ratio, time counting, automated instruments. Please contact BIOLABO technical
calibration factor. support.
4. If control is still out of range, use a new vial of reagent and assay
again
5. If control is still out of range, please contact BIOLABO technical CALCULATION
support or your local Agent.
Calculate the result as follows:
EXPECTED VALUES (5)
With theoretical factor:
Adult GGT activity, measured at 37°C
∆Abs./min.) x 2121
IU/L = (∆
Men (IU/L) 11-50
µkat/L = IU/L
Women (IU/L) 7-32
60
Each laboratory should establish its own normal ranges for the
population it serves.
With serous multicalibrator
GGT Activity = (∆Abs/min) Assay x Calibrator Concentration
PERFORMANCE CHARACTERISTICS (∆Abs/min) Calibrator
Tests Results on Kenza 240 TX:
REFERENCES
Within-run Normal Medium High Run to run Normal Medium High
(1) TIETZ N.W. Text book of clinical chemistry, 3rd Ed. C.A. Burtis, E.R.
N = 30 level level level N = 30 level level level Ashwood, W.B. Saunders (1999) p. 686-689.
Mean UI/L 43 104 329 Mean UI/L 46 111 363 (2) Clinical Guide to Laboratory Test, 4th Ed., N.W. TIETZ (2006) p. 470-473..
th
(3) YOUNG D.S., Effect of Drugs on Clinical laboratory Tests, 4 Ed. (1995) p.
S.D. UI/L 0.9 0.7 3.6 S.D.UI/L 1.3 2.5 10.8 3-296 à 3-300
C.V% 2.0 0.6 1.1 C.V% 2.7 2.3 3 (4) SZASZ G., Clin. Chem., (1969), 15, p.124
(5) SZASZ G., Bergmeyer H.U.,ed. Methods of Enzymatic analysis, (1974)
Criteria < 4.5% < 4.5% < 4% Criteria < 6% < 6% < 5% Weinheim Verlag Chemie
Detection limit: environ 4 UI/L
Sensitivity for 21 UI/L: environ 0,010 ∆Abs/min
Comparison studies with commercially available reagent:
y = 0.9387 x +2.214 r = 0,9985
LINEARITY
The assay is linear up to 320 IU/L (5.33 µkat/L).
Above, dilute specimen with saline solution and assay again taking into
account the dilution factor. Linearity will depend on the
specimen/reagent ratio.
IVD REF LOT →
Manufacturer Use by In vitro diagnostic Temperature limitation Catalogue number See insert Batch number Store away from light sufficient for dilute with
Made in France Latest revision : www.biolabo.fr Revision : 19/06/2013
You might also like
- GAMMA-GT Carboxy GPNA: BiolaboDocument2 pagesGAMMA-GT Carboxy GPNA: BiolaboFariz KasyidiNo ratings yet
- at Gamma GTDocument2 pagesat Gamma GTNia MarianiNo ratings yet
- GGT enDocument2 pagesGGT enKaoueche OmarNo ratings yet
- GGT InsertDocument2 pagesGGT InsertsharmashyamsinghNo ratings yet
- Alkaline Phosphatase Activity Test KitDocument2 pagesAlkaline Phosphatase Activity Test KitRury Darwa Ningrum100% (1)
- 1126005I Rev. en KR 04Document2 pages1126005I Rev. en KR 04Nguyễn HuynhNo ratings yet
- Reagen Kit DiasysDocument2 pagesReagen Kit DiasysNiken CahyaningrumNo ratings yet
- ALT GPT (IFCC) Single Vial: BiolaboDocument2 pagesALT GPT (IFCC) Single Vial: Biolabowindy ajengNo ratings yet
- Multiparametric Calibrator: Biolabo MulticalibratorDocument1 pageMultiparametric Calibrator: Biolabo MulticalibratorFariz KasyidiNo ratings yet
- Amilase PDFDocument2 pagesAmilase PDFFariz KasyidiNo ratings yet
- GGT FluitestDocument4 pagesGGT FluitestCristian LaraNo ratings yet
- EN GGT BAOSR6x19 USDocument2 pagesEN GGT BAOSR6x19 USDharmesh PatelNo ratings yet
- PI e BIL - DIRECT 15Document2 pagesPI e BIL - DIRECT 15Nia HidmahNo ratings yet
- Liquick Cor-GGT: Diagnostic Kit For Determination of - Glutamyltransferase ActivityDocument2 pagesLiquick Cor-GGT: Diagnostic Kit For Determination of - Glutamyltransferase ActivityIvana BajunovicNo ratings yet
- Kit InsertDocument2 pagesKit InsertfaujraneNo ratings yet
- At 80027Document2 pagesAt 80027Yesha Bio ValenciaNo ratings yet
- Alkaline Phosphatase FS : Order Information SpecimenDocument3 pagesAlkaline Phosphatase FS : Order Information SpecimenmnemonicsNo ratings yet
- Kit Insert Magnesium (Calmagite)Document2 pagesKit Insert Magnesium (Calmagite)rai citaNo ratings yet
- BioSystems GGTDocument1 pageBioSystems GGTIvana BajunovicNo ratings yet
- TOTAL PROTEIN Biuret Method: BiolaboDocument2 pagesTOTAL PROTEIN Biuret Method: BiolaboVenura VishwajithNo ratings yet
- Creatinine: Kinetic MethodDocument2 pagesCreatinine: Kinetic MethodVenura VishwajithNo ratings yet
- ALT - (GPT) Test Adv. Clinical BiochemicalDocument2 pagesALT - (GPT) Test Adv. Clinical BiochemicalAli AlhamdaniNo ratings yet
- At 80009Document2 pagesAt 80009Abrian Aziz KNo ratings yet
- GGT 110 - 330 XL-1000 - Xsys0011 - 77 - HDocument4 pagesGGT 110 - 330 XL-1000 - Xsys0011 - 77 - HMatibar RahmanNo ratings yet
- Pglu-Pap GB-D 21 001Document4 pagesPglu-Pap GB-D 21 001akaweadeNo ratings yet
- Chloride: Colorimetric MethodDocument2 pagesChloride: Colorimetric MethodFariz KasyidiNo ratings yet
- Pi e LDH 21 Ifcc 3Document2 pagesPi e LDH 21 Ifcc 3Osama Ben DawNo ratings yet
- CLONATEST ALT/GPT opt. performance and characteristicsDocument2 pagesCLONATEST ALT/GPT opt. performance and characteristicsthureinwinnNo ratings yet
- PHP UYjhb 7Document2 pagesPHP UYjhb 7BerhaneNo ratings yet
- GA4231 00 - Total BilirubinDocument2 pagesGA4231 00 - Total BilirubinTrần Thanh Viện100% (1)
- Protéines Totales Biuret - ENDocument2 pagesProtéines Totales Biuret - ENYousra NanoNo ratings yet
- Creatinine: Kinetic MethodDocument2 pagesCreatinine: Kinetic MethodCarina AngelNo ratings yet
- IFU - R920 e GGT 7Document3 pagesIFU - R920 e GGT 7Osama Ben DawNo ratings yet
- Principle of The Method Quality Control: Alkaline PicrateDocument1 pagePrinciple of The Method Quality Control: Alkaline PicrateRisqon Anjahiranda Adiputra100% (1)
- L.D.H. (LDH-P) : SFBC Modified MethodDocument2 pagesL.D.H. (LDH-P) : SFBC Modified MethodFariz KasyidiNo ratings yet
- Ifu BX e Bil Total 1Document6 pagesIfu BX e Bil Total 1Aditya FerdyNo ratings yet
- Protap - Amylase (Dyasis)Document2 pagesProtap - Amylase (Dyasis)TEMPORALIS 2018No ratings yet
- Alat (GPT) Fs : Order Information Warnings and PrecautionsDocument3 pagesAlat (GPT) Fs : Order Information Warnings and Precautionssovi haswindhaNo ratings yet
- Clonatest Sodium BRDocument4 pagesClonatest Sodium BRSuprovet LabotatorioNo ratings yet
- AMYLASEDocument2 pagesAMYLASEfqahNo ratings yet
- SGPT (ALT) - Kit InsertDocument2 pagesSGPT (ALT) - Kit InsertDharmesh Patel100% (1)
- IFU - R910 e CREA - JAFFE 14Document3 pagesIFU - R910 e CREA - JAFFE 14HaisNo ratings yet
- Sodium: Enzymatic MethodDocument2 pagesSodium: Enzymatic MethodFariz KasyidiNo ratings yet
- Clonatest Alt/Gpt OptDocument4 pagesClonatest Alt/Gpt OptSuprovet LabotatorioNo ratings yet
- Asat (Got) Fs : Order Information Warnings and PrecautionsDocument6 pagesAsat (Got) Fs : Order Information Warnings and PrecautionsTesya PratiwiNo ratings yet
- PI e UREA 17Document2 pagesPI e UREA 17sovi haswindhaNo ratings yet
- IFU - BX e CREA - JAFFE 1Document10 pagesIFU - BX e CREA - JAFFE 1Agnihotram GopinathNo ratings yet
- IFU - R910 e ALT 2Document4 pagesIFU - R910 e ALT 2Osama Ben DawNo ratings yet
- Total Protein Test ProcedureDocument2 pagesTotal Protein Test ProcedureTrần Thanh ViệnNo ratings yet
- Alat (GPT) Fs : Order Information Warnings and PrecautionsDocument4 pagesAlat (GPT) Fs : Order Information Warnings and Precautionssovi haswindhaNo ratings yet
- Ifu Iga 41 14Document5 pagesIfu Iga 41 14Wesam TayaNo ratings yet
- AT 22010 HbA1cDocument2 pagesAT 22010 HbA1cMohammed R.HusseinNo ratings yet
- IFU R920-e-LDH 21 IFCC-3Document3 pagesIFU R920-e-LDH 21 IFCC-3Osama Ben DawNo ratings yet
- PI e BIL - TOTAL 16Document2 pagesPI e BIL - TOTAL 16ilhamNo ratings yet
- Clinical ChemistryDocument38 pagesClinical Chemistryxox18No ratings yet
- PI e AST 2Document3 pagesPI e AST 2Cindy Mae A. PogoyNo ratings yet
- at Bili DmsoDocument2 pagesat Bili DmsoP VijayaNo ratings yet
- BioDocument3 pagesBioمحمد رحيم حسن محمودNo ratings yet
- Degradation OF Glucose 6-Phosphate Dehydrogenase Into Forms BY TrypsinDocument5 pagesDegradation OF Glucose 6-Phosphate Dehydrogenase Into Forms BY Trypsinمحمد رحيم حسن محمودNo ratings yet
- Who To Write Medical ThesisDocument28 pagesWho To Write Medical Thesisمحمد رحيم حسن محمودNo ratings yet
- Relationship Between Levels of Fasting Blood Glucose and Hba1C in Prediabetes PatientsDocument4 pagesRelationship Between Levels of Fasting Blood Glucose and Hba1C in Prediabetes Patientsمحمد رحيم حسن محمودNo ratings yet
- Theory Time Table - MorningDocument1 pageTheory Time Table - Morningمحمد رحيم حسن محمودNo ratings yet
- Non-Pathogenic Amoeba - Free LivingDocument42 pagesNon-Pathogenic Amoeba - Free Livingمحمد رحيم حسن محمودNo ratings yet
- Correlation Between Fasting Blood Sugar Level and HbA1C Levels in Type 2 Diabetes Mellitus PatientsDocument29 pagesCorrelation Between Fasting Blood Sugar Level and HbA1C Levels in Type 2 Diabetes Mellitus Patientsمحمد رحيم حسن محمودNo ratings yet
- Elisa: Alkitab University - Collage of Medical Techniques Department of Medical AnalysisDocument9 pagesElisa: Alkitab University - Collage of Medical Techniques Department of Medical Analysisمحمد رحيم حسن محمودNo ratings yet
- Stool Examination (4)Document29 pagesStool Examination (4)محمد رحيم حسن محمودNo ratings yet
- Theory Time Table - MorningDocument1 pageTheory Time Table - Morningمحمد رحيم حسن محمودNo ratings yet
- Laboratory No. 13 Title: Clostridium Tetani: Al-Kitab University College Medical Analysis TechniqueDocument5 pagesLaboratory No. 13 Title: Clostridium Tetani: Al-Kitab University College Medical Analysis Techniqueمحمد رحيم حسن محمودNo ratings yet
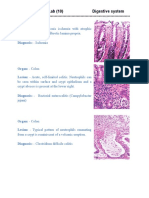
- Histopathology-Lab (10) Digestive SystemDocument2 pagesHistopathology-Lab (10) Digestive Systemمحمد رحيم حسن محمودNo ratings yet
- Antibiotic Susceptibility TestsDocument18 pagesAntibiotic Susceptibility Testsمحمد رحيم حسن محمودNo ratings yet
- Theory Time Table - EveningDocument1 pageTheory Time Table - Eveningمحمد رحيم حسن محمودNo ratings yet
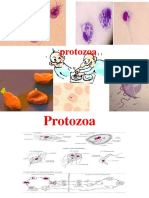
- ProtozoaDocument57 pagesProtozoaمحمد رحيم حسن محمودNo ratings yet
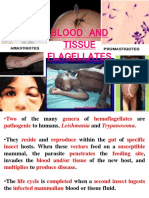
- Hemofl - AgellatesDocument46 pagesHemofl - Agellatesمحمد رحيم حسن محمودNo ratings yet
- Trichomonas 2021Document31 pagesTrichomonas 2021محمد رحيم حسن محمود100% (1)
- Seconed Term ExaminationDocument8 pagesSeconed Term Examinationمحمد رحيم حسن محمودNo ratings yet
- Top 5 Anticoagulants Used in Hematology Laboratory - BiologyDocument26 pagesTop 5 Anticoagulants Used in Hematology Laboratory - Biologyمحمد رحيم حسن محمودNo ratings yet
- Ast Got: (IFCC) Single VialDocument2 pagesAst Got: (IFCC) Single Vialwindy ajengNo ratings yet
- High Level of Individual Lipid Profile and Lipid RatioDocument9 pagesHigh Level of Individual Lipid Profile and Lipid Ratioمحمد رحيم حسن محمودNo ratings yet
- Histopathology-Lab (8) Digestive SystemDocument2 pagesHistopathology-Lab (8) Digestive Systemمحمد رحيم حسن محمودNo ratings yet
- Lab Examination 2nd Term 2021Document7 pagesLab Examination 2nd Term 2021محمد رحيم حسن محمودNo ratings yet
- ALT - (GPT) Test Adv. Clinical BiochemicalDocument2 pagesALT - (GPT) Test Adv. Clinical BiochemicalAli AlhamdaniNo ratings yet
- Histopathology-Lab (8) Digestive SystemDocument2 pagesHistopathology-Lab (8) Digestive Systemمحمد رحيم حسن محمودNo ratings yet
- Anaerobic Specimen Collection and TransportDocument22 pagesAnaerobic Specimen Collection and Transportمحمد رحيم حسن محمودNo ratings yet
- Hematological Changes in Pregnancy The PreparatiDocument6 pagesHematological Changes in Pregnancy The Preparatiمحمد رحيم حسن محمودNo ratings yet
- Total Protein Test by MDDocument6 pagesTotal Protein Test by MDمحمد رحيم حسن محمودNo ratings yet
- (Clinical Chemistry) : TriglyceridesDocument5 pages(Clinical Chemistry) : Triglyceridesمحمد رحيم حسن محمودNo ratings yet
- Outcome of Conservative Therapy in COVID-19 in Pts Presenting With GI BleedingDocument7 pagesOutcome of Conservative Therapy in COVID-19 in Pts Presenting With GI BleedingTAUSEEFA JANNo ratings yet
- The Effects of Hypoalbuminaemia On Optimizing.3Document12 pagesThe Effects of Hypoalbuminaemia On Optimizing.3ismaelito22No ratings yet
- SNS 1 (Circulation)Document7 pagesSNS 1 (Circulation)Dheeraj Kumar100% (1)
- Patologia Inflamatorie A Sânului: DR Panuța AndrianDocument31 pagesPatologia Inflamatorie A Sânului: DR Panuța AndrianPanuta AndrianNo ratings yet
- ChlorpheniramineDocument6 pagesChlorpheniramineÇhämäñ ÇhNo ratings yet
- Clsi Catalog 2021 WebDocument34 pagesClsi Catalog 2021 WebPaul BandaNo ratings yet
- PDF COPY The Use of Icing Sugar To Reduce The Oedematous StomaDocument16 pagesPDF COPY The Use of Icing Sugar To Reduce The Oedematous StomaIsa HaironiNo ratings yet
- Blood Brain Barrier BBBDocument10 pagesBlood Brain Barrier BBBaamnaNo ratings yet
- Nursing Management of AmoebiasisDocument2 pagesNursing Management of Amoebiasisjiedysy100% (1)
- 704 Viet Nam Fact SheetsDocument2 pages704 Viet Nam Fact SheetsKhánh NgôNo ratings yet
- The Essential Keto CookbookDocument7 pagesThe Essential Keto CookbookTarakanta PatraNo ratings yet
- Clinical Efficacy and Safety of Meropenem in The Treatment of Severe Neonatal Bacterial Infectious PneumoniaDocument6 pagesClinical Efficacy and Safety of Meropenem in The Treatment of Severe Neonatal Bacterial Infectious PneumoniaI Made AryanaNo ratings yet
- RP-HPLC Method of The Estimation of Folic Acid in TabletsDocument5 pagesRP-HPLC Method of The Estimation of Folic Acid in Tabletsminela dacaNo ratings yet
- Nota Dan Amali Anatomi Dan Fisiologi - 3 - Nota Jantung & Sis. KardiovaskularDocument32 pagesNota Dan Amali Anatomi Dan Fisiologi - 3 - Nota Jantung & Sis. KardiovaskularFadilla LayangNo ratings yet
- Why Is A Prothrombin Time Test Performed?: WarfarinDocument6 pagesWhy Is A Prothrombin Time Test Performed?: WarfarinMamta ShindeNo ratings yet
- J2011.Decompressive Craniectomy Technical NoteDocument6 pagesJ2011.Decompressive Craniectomy Technical NoteKysy CodonNo ratings yet
- Mod 3Document16 pagesMod 3Gin CruzNo ratings yet
- Abatacept For Treatment of Adults Hospitalized With Moderate or Severe CovidDocument3 pagesAbatacept For Treatment of Adults Hospitalized With Moderate or Severe CovidHoracioArizaNo ratings yet
- CVS Drugs: Antihypertensives, Diuretics, Anti-AnginalsDocument21 pagesCVS Drugs: Antihypertensives, Diuretics, Anti-AnginalsAudrey Beatrice ReyesNo ratings yet
- BIO202L Lab 16 Upload DocumentDocument9 pagesBIO202L Lab 16 Upload Documentzape limitedNo ratings yet
- Relief OCD: A Guide For People With Obsessive Compulsive DisorderDocument18 pagesRelief OCD: A Guide For People With Obsessive Compulsive DisorderRadu CzechNo ratings yet
- CASE REPORT Laser-Assisted Treatment of SialolithiasisDocument2 pagesCASE REPORT Laser-Assisted Treatment of SialolithiasisPeter SalimNo ratings yet
- ICU Management of COVID Patients: PreventionDocument13 pagesICU Management of COVID Patients: PreventionYuri SadewoNo ratings yet
- Trauma GinjalDocument26 pagesTrauma GinjalMyra MieraNo ratings yet
- 14 GHSA Progress Report September 2022 March 2023Document30 pages14 GHSA Progress Report September 2022 March 2023Obo KeroNo ratings yet
- Anacardiaceae Family Homeopathy Remedies - Homeopathy ResourceDocument8 pagesAnacardiaceae Family Homeopathy Remedies - Homeopathy ResourceMuhammad ArshadNo ratings yet
- Difference Between Monoclonal and Polyclonal Antibodies: October 2017Document8 pagesDifference Between Monoclonal and Polyclonal Antibodies: October 2017Everton MonteiroNo ratings yet
- Microneedling & Nanopore PresentationDocument58 pagesMicroneedling & Nanopore PresentationkatherineNo ratings yet
- Gage JR 2001 An Update On The Treatment of Gait Problems in CPDocument10 pagesGage JR 2001 An Update On The Treatment of Gait Problems in CPMarco Tulio FigueroaNo ratings yet
- Effects of Community Based Rehabilitation-DraftDocument2 pagesEffects of Community Based Rehabilitation-DraftJayson V. CastroNo ratings yet