Professional Documents
Culture Documents
Nitrogen Oxide
Uploaded by
Kit Lbj0 ratings0% found this document useful (0 votes)
29 views1 pageNitrogen oxides are compounds produced during combustion from vehicle exhaust and burning fossil fuels. They are emitted from power plants, vehicles, gas stoves, and burning wood. Excess nitrogen oxides can damage and kill plants, crops, and biological systems. They contribute to acid rain and smog formation and are part of the nitrogen cycle in air, soil, and water.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentNitrogen oxides are compounds produced during combustion from vehicle exhaust and burning fossil fuels. They are emitted from power plants, vehicles, gas stoves, and burning wood. Excess nitrogen oxides can damage and kill plants, crops, and biological systems. They contribute to acid rain and smog formation and are part of the nitrogen cycle in air, soil, and water.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
29 views1 pageNitrogen Oxide
Uploaded by
Kit LbjNitrogen oxides are compounds produced during combustion from vehicle exhaust and burning fossil fuels. They are emitted from power plants, vehicles, gas stoves, and burning wood. Excess nitrogen oxides can damage and kill plants, crops, and biological systems. They contribute to acid rain and smog formation and are part of the nitrogen cycle in air, soil, and water.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
NITROGEN
OXIDE
Nitrogen Oxides are compounds of nitrogen and oxygen
produced during combustion. They are emitted from
vehicle exhaust, and the burning of coal, oil, diesel fuel,
and natural gas, especially from electric power plants.
They are also emitted by cigarettes, gas stoves, kerosene
heaters, wood burning etc.
Chemical Properties: Environmental Effects:
*NO only burns when Description
heated with hydrogen, Excessive levels of the oxides of nitrogen,
and forms nitric acid (a particularly nitrogen dioxide (NO2), can cause
strong acid) when death in plants and roots and damage the leaves of
dissolved in water. many agricultural crops. NO2 is the damaging
*Colorless, odorless gas component of photochemical smog. Excessive
that is used as an levels increase the acidity of rain (lower the pH),
anesthetic and and thus lower the pH of surface and ground
analgesic. High waters and soil. The lowered pH can have harmful
concentrations cause a effects, possibly even death, on a variety of
narcotic effect and may biological systems.
replace oxygen, causing Entering the environment
death by asphyxia. Oxides of nitrogen are part of the biogeochemical
Nitrous oxide is also cycling of nitrogen, and are found in air, soil and
used as a food aerosol water.
in the preparation of In the atmosphere, the oxides of nitrogen are
whipping cream. rapidly equilibrated to nitrogen dioxide (NO2),
*A naturally occurring which eventually forms acid rain. In the
gas that is colorless and stratosphere, oxides of nitrogen play a crucial role
non flammable. It can in maintaining the levels of ozone. Ozone is formed
be manufactured and through the photochemical reaction between
used for a variety of nitrogen dioxide and oxygen.
things such as a Where it ends up
pharmacologic agent to Oxides of nitrogen are rapidly broken down by
produce anesthesia, a reacting with other substances found in the air.
food additive as a Nitrogen dioxide can form nitric acid in sunlight,
propellant, and an and is a major constituent of acid rain,
additive to fuels to tropospheric ozone and smog. Nitrogen oxides
increase available
react in the soil and the water to nitric acid.
oxygen in combustion.
You might also like
- Top 10 Most Powerful Openings in Chess PDFDocument12 pagesTop 10 Most Powerful Openings in Chess PDFsyaf file gwNo ratings yet
- Lecture6 Nitrogen PDFDocument55 pagesLecture6 Nitrogen PDFgagileNo ratings yet
- The 5 Nutrient Cycles - Science Book 3rd Grade | Children's Science Education booksFrom EverandThe 5 Nutrient Cycles - Science Book 3rd Grade | Children's Science Education booksNo ratings yet
- Short-Term Memory and Working MemoryDocument32 pagesShort-Term Memory and Working Memorysiempreviva84No ratings yet
- Grade 10 - SharmajiDocument8 pagesGrade 10 - Sharmajijuned ansariNo ratings yet
- Oblicon NotesDocument14 pagesOblicon NotesCee Silo Aban100% (1)
- Triple Net Lease Research Report by The Boulder GroupDocument2 pagesTriple Net Lease Research Report by The Boulder GroupnetleaseNo ratings yet
- How To Become An Excellent Student: Pay AttentionDocument2 pagesHow To Become An Excellent Student: Pay AttentionKit LbjNo ratings yet
- Fun Facts about Nitrogen : Chemistry for Kids The Element Series | Children's Chemistry BooksFrom EverandFun Facts about Nitrogen : Chemistry for Kids The Element Series | Children's Chemistry BooksNo ratings yet
- Environmental ChemistryDocument40 pagesEnvironmental ChemistryharryNo ratings yet
- 2003catalog PDFDocument25 pages2003catalog PDFPH "Pete" PetersNo ratings yet
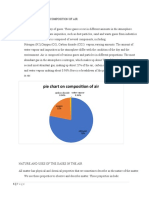
- Composition of Air Grade 8 NotesDocument5 pagesComposition of Air Grade 8 NotesFaithNo ratings yet
- Acid RainDocument11 pagesAcid RainTEJAS JAINNo ratings yet
- EE Lab 3Document4 pagesEE Lab 3Muhammad AbdullahNo ratings yet
- Science Note 1Document2 pagesScience Note 1Insha EhsanNo ratings yet
- Acid RainDocument6 pagesAcid RainАнастасия МелешкоNo ratings yet
- Acid Rain-The Major Cause of Pollution: Its Causes, Effects: Subodh KumarDocument6 pagesAcid Rain-The Major Cause of Pollution: Its Causes, Effects: Subodh KumarShruthi GNo ratings yet
- 5 NOx-SOxDocument2 pages5 NOx-SOxmy printNo ratings yet
- Non MetalsDocument19 pagesNon Metalscoliciastapleton981No ratings yet
- 9.3 - Section 2Document51 pages9.3 - Section 2Joseph WongNo ratings yet
- Atmospheric Pollution: Environmental ChemistryDocument9 pagesAtmospheric Pollution: Environmental Chemistryminhazalam786No ratings yet
- Overview, Types, Sources & Control MeasuresDocument43 pagesOverview, Types, Sources & Control Measuressiti surayaNo ratings yet
- Assignment No#1: Department of Environmental SciencesDocument9 pagesAssignment No#1: Department of Environmental SciencesIbrahim AliNo ratings yet
- Air Pollution FinalDocument23 pagesAir Pollution Finalnamansehgal3006No ratings yet
- Unit - I Introduction PollutionDocument9 pagesUnit - I Introduction PollutionNaveen AshraeNo ratings yet
- 7 1+Acid+DepositionDocument17 pages7 1+Acid+DepositionSanchanaNo ratings yet
- Air Pollution-2021-22Document51 pagesAir Pollution-2021-22AshwiniNo ratings yet
- Science The Air Around Us: FolioDocument21 pagesScience The Air Around Us: FolioAfiqah NurhabreyahNo ratings yet
- Green House Gasses and Acid RainDocument11 pagesGreen House Gasses and Acid RainDog GodNo ratings yet
- Ncert 11 ChemistryDocument11 pagesNcert 11 ChemistryTr Mazhar PunjabiNo ratings yet
- MuhojaDocument6 pagesMuhojaSire MkubwaNo ratings yet
- Acid Rain Causes Effects and Remedicition Real WorkDocument36 pagesAcid Rain Causes Effects and Remedicition Real WorkAurelia HernandezNo ratings yet
- Chemistry Project (1) (Mohamed Part)Document6 pagesChemistry Project (1) (Mohamed Part)mohamed amirNo ratings yet
- AIR QUALITY AND POLLUTION (TKA 3301) LECTURE NOTES 9 - Criteria Pollutants (NOx, SOx, O3)Document44 pagesAIR QUALITY AND POLLUTION (TKA 3301) LECTURE NOTES 9 - Criteria Pollutants (NOx, SOx, O3)mamat88No ratings yet
- W13 NOx ControlDocument43 pagesW13 NOx Controlmr styloNo ratings yet
- Inorganic Chemistry Class - 11 Oxygen by Arun Dahal (AD) Lecture-1Document16 pagesInorganic Chemistry Class - 11 Oxygen by Arun Dahal (AD) Lecture-1Bhuwan GhimireNo ratings yet
- Environmental Chemistry: Atmospheric PollutionDocument6 pagesEnvironmental Chemistry: Atmospheric PollutiongnkstarNo ratings yet
- Acid Rain Description and Analysis: TreeboxDocument6 pagesAcid Rain Description and Analysis: TreeboxNanthini RajanderanNo ratings yet
- Fiitjee Viii 05 Pollution of Air and Water-1Document96 pagesFiitjee Viii 05 Pollution of Air and Water-1yashNo ratings yet
- Environmental Chemistry - KPDocument4 pagesEnvironmental Chemistry - KPKiran KiruNo ratings yet
- 8.5 Acid DepositionDocument33 pages8.5 Acid DepositionElsa MahardikaNo ratings yet
- Environmental Chemistry (Air)Document32 pagesEnvironmental Chemistry (Air)Hussain HashmiNo ratings yet
- 1 AirDocument51 pages1 AirAli HarbNo ratings yet
- Chapter 14 Nitrogen and Sulphur (Chemistry AS - Level)Document3 pagesChapter 14 Nitrogen and Sulphur (Chemistry AS - Level)Mohamed AkkashNo ratings yet
- Acid Rain - PP - Watermark PDFDocument15 pagesAcid Rain - PP - Watermark PDFAbhishek GuptaNo ratings yet
- Acid Rain Cause & Prevention: Name Student IdDocument9 pagesAcid Rain Cause & Prevention: Name Student IdNur AsyikinNo ratings yet
- Acid RainDocument3 pagesAcid RainNiken RumaniNo ratings yet
- AIR NotesDocument5 pagesAIR NotesjpkaomeNo ratings yet
- Enviromental IssuesDocument80 pagesEnviromental IssuesNAYAN BISWASNo ratings yet
- CARBON COMPOUNDS: Pollution Aspects: Received Date: Jan. 2020 Revised: April 2020 Accepted: June 2020Document9 pagesCARBON COMPOUNDS: Pollution Aspects: Received Date: Jan. 2020 Revised: April 2020 Accepted: June 2020Vaibhav SiddharthNo ratings yet
- Environmental PollutionDocument11 pagesEnvironmental PollutionrupeshNo ratings yet
- ICSE Selina Solution For Class 9 Chemistry Chapter 8 Exercise QuestionsDocument16 pagesICSE Selina Solution For Class 9 Chemistry Chapter 8 Exercise QuestionsYash KapoorNo ratings yet
- Nitrogen Oxides Formation From Automobile Exhausts: & It'S Harmful Effects To Human and EnvironmentDocument23 pagesNitrogen Oxides Formation From Automobile Exhausts: & It'S Harmful Effects To Human and Environmentreymar ungabNo ratings yet
- 5512 Et EtDocument14 pages5512 Et EtSayed Newaj ChowdhuryNo ratings yet
- Toxic Air PollutantsDocument2 pagesToxic Air PollutantsprimewaterNo ratings yet
- Environmental Chemistry: Chapter - 16Document9 pagesEnvironmental Chemistry: Chapter - 16Haa KksakNo ratings yet
- Clean Air Is Composed of ApproximatelyDocument3 pagesClean Air Is Composed of ApproximatelyMbotake LawsonNo ratings yet
- Chapter 10 - Toxic GasesDocument81 pagesChapter 10 - Toxic GasesĐặng Ngọc Châu VyNo ratings yet
- Q1. Write Briefly On Composition of Atmosphere Ans.: It Is Caused by Burning Fossil Fuels, Like Coal and PetroleumDocument6 pagesQ1. Write Briefly On Composition of Atmosphere Ans.: It Is Caused by Burning Fossil Fuels, Like Coal and PetroleumRonnith NandyNo ratings yet
- Chemisty Project - 9 - F - 100383 - Acid Rain (Autosaved)Document8 pagesChemisty Project - 9 - F - 100383 - Acid Rain (Autosaved)Lakshya BhatiNo ratings yet
- CIE Chemistry A Level: 13: Nitrogen and SulfurDocument4 pagesCIE Chemistry A Level: 13: Nitrogen and Sulfurbubutrain2003No ratings yet
- N CycleDocument2 pagesN CycleKhiro CapiliNo ratings yet
- Generally: What Is Acid Rain: Key Pointss!!!Document4 pagesGenerally: What Is Acid Rain: Key Pointss!!!aihpendoyNo ratings yet
- Environmental ChemistryDocument19 pagesEnvironmental ChemistryNeeraj RathiNo ratings yet
- ChemistryDocument5 pagesChemistrySathish N TNo ratings yet
- The Chemistry of Photo Chemical SmogDocument10 pagesThe Chemistry of Photo Chemical Smogtracx100% (2)
- Atmosphere & environment-OL-NotesDocument4 pagesAtmosphere & environment-OL-Notesshlaibat13No ratings yet
- Science A PT Self CheckDocument6 pagesScience A PT Self CheckKit LbjNo ratings yet
- Orel Cardenas A2Document1 pageOrel Cardenas A2Kit LbjNo ratings yet
- 3 Importance of Igneous Rock Common Igneous Rock and Their ImportanceDocument16 pages3 Importance of Igneous Rock Common Igneous Rock and Their ImportanceKit LbjNo ratings yet
- PPA FOR PRELIMS (OA) 2nd SEM 2023-24Document2 pagesPPA FOR PRELIMS (OA) 2nd SEM 2023-24Kit LbjNo ratings yet
- Orel FittreflectionDocument1 pageOrel FittreflectionKit LbjNo ratings yet
- Chapter 3 and QuestionnaireDocument5 pagesChapter 3 and QuestionnaireKit LbjNo ratings yet
- CE 2131 - TLO2 - Mineralogy Part2Document8 pagesCE 2131 - TLO2 - Mineralogy Part2Kit LbjNo ratings yet
- Esguerra Module 2 FitDocument3 pagesEsguerra Module 2 FitKit LbjNo ratings yet
- CFE 101 - Module 1 - MIssionary ResponseDocument3 pagesCFE 101 - Module 1 - MIssionary ResponseKit LbjNo ratings yet
- Explain Module 10 Transportation EngineeringDocument2 pagesExplain Module 10 Transportation EngineeringKit LbjNo ratings yet
- Updated Nutrition Spreadsheet (With Workout Tracker)Document54 pagesUpdated Nutrition Spreadsheet (With Workout Tracker)Kit LbjNo ratings yet
- Edited FW NO. 7 GROUP REPORTDocument7 pagesEdited FW NO. 7 GROUP REPORTKit LbjNo ratings yet
- Elaborate Module 8 Water Resources Engineering Concepts and StructuresDocument2 pagesElaborate Module 8 Water Resources Engineering Concepts and StructuresKit LbjNo ratings yet
- Oficiar Dan Isaac P. FIT HW Module 2 Unit 2Document1 pageOficiar Dan Isaac P. FIT HW Module 2 Unit 2Kit LbjNo ratings yet
- Esguerra Module 2 FitDocument3 pagesEsguerra Module 2 FitKit LbjNo ratings yet
- Dot3 BastoDocument4 pagesDot3 BastoKit LbjNo ratings yet
- Dot 2 BastoDocument5 pagesDot 2 BastoKit LbjNo ratings yet
- Oficiar Dan Isaac P. FIT - HM Modul 2 Unit 1Document2 pagesOficiar Dan Isaac P. FIT - HM Modul 2 Unit 1Kit LbjNo ratings yet
- Paradigm ShiftDocument1 pageParadigm ShiftKit LbjNo ratings yet
- GSTS CompilationDocument5 pagesGSTS CompilationKit LbjNo ratings yet
- RVE Putting God First 2Document1 pageRVE Putting God First 2Kit LbjNo ratings yet
- School of Engineering and Architecture: Saint Louis University Rubric For Enggphys Laboratory ReportsDocument1 pageSchool of Engineering and Architecture: Saint Louis University Rubric For Enggphys Laboratory ReportsKit LbjNo ratings yet
- Medieval PeriodDocument1 pageMedieval PeriodKit LbjNo ratings yet
- 1stYrCollege 1st Sem SchedDocument3 pages1stYrCollege 1st Sem SchedKit LbjNo ratings yet
- DemoticDocument1 pageDemoticKit LbjNo ratings yet
- GOUSGOUNIS Anastenaria & Transgression of The SacredDocument14 pagesGOUSGOUNIS Anastenaria & Transgression of The Sacredmegasthenis1No ratings yet
- Scrabble Scrabble Is A Word Game in Which Two or Four Players Score Points by Placing Tiles, EachDocument4 pagesScrabble Scrabble Is A Word Game in Which Two or Four Players Score Points by Placing Tiles, EachNathalie Faye De PeraltaNo ratings yet
- MDL ChallanDocument1 pageMDL ChallanPratik V PaliwalNo ratings yet
- Revised Poea Rules and Regulations Governing RecruitmentDocument8 pagesRevised Poea Rules and Regulations Governing RecruitmenthansNo ratings yet
- 1167 Nine Planets in TamilDocument1 page1167 Nine Planets in TamilmanijaiNo ratings yet
- Question QP MCQ A BDocument60 pagesQuestion QP MCQ A BPrashant JhaNo ratings yet
- BZU Ad 31 12 12Document15 pagesBZU Ad 31 12 12Saleem MirraniNo ratings yet
- Banana Oil LabDocument5 pagesBanana Oil LabjbernayNo ratings yet
- Stock Control Management SyestemDocument12 pagesStock Control Management SyestemJohn YohansNo ratings yet
- (2010) Formulaic Language and Second Language Speech Fluency - Background, Evidence and Classroom Applications-Continuum (2010)Document249 pages(2010) Formulaic Language and Second Language Speech Fluency - Background, Evidence and Classroom Applications-Continuum (2010)Như Đặng QuếNo ratings yet
- Foundation Plan: Scale 1:100 MTSDocument1 pageFoundation Plan: Scale 1:100 MTSJayson Ayon MendozaNo ratings yet
- Silent Reading With Graph1Document2 pagesSilent Reading With Graph1JonaldSamueldaJoseNo ratings yet
- Tazkira Mujaddid AlfesaniDocument53 pagesTazkira Mujaddid AlfesanisayedNo ratings yet
- Carr v. NH State Prison, Warden - Document No. 2Document5 pagesCarr v. NH State Prison, Warden - Document No. 2Justia.comNo ratings yet
- Research Poster 1Document1 pageResearch Poster 1api-662489107No ratings yet
- Stop Motion RubricDocument3 pagesStop Motion Rubricapi-506782994No ratings yet
- Resonant Excitation of Coherent Cerenkov Radiation in Dielectric Lined WaveguidesDocument3 pagesResonant Excitation of Coherent Cerenkov Radiation in Dielectric Lined WaveguidesParticle Beam Physics LabNo ratings yet
- Grade Up CurrentsDocument273 pagesGrade Up CurrentsAmiya RoyNo ratings yet
- Cambridge IGCSE: PHYSICS 0625/63Document16 pagesCambridge IGCSE: PHYSICS 0625/63...No ratings yet
- Entrep 1st PerioDocument5 pagesEntrep 1st PerioMargarette FajardoNo ratings yet
- ReferencesDocument12 pagesReferencesBilal RazzaqNo ratings yet
- Micro Fibra Sintetica at 06-MapeiDocument2 pagesMicro Fibra Sintetica at 06-MapeiSergio GonzalezNo ratings yet
- Vince - Michael - Intermediate - Language Assive 1Document5 pagesVince - Michael - Intermediate - Language Assive 1Николай КолевNo ratings yet
- Schedule 1 Allison Manufacturing Sales Budget For The Quarter I Ended March 31 First QuarterDocument16 pagesSchedule 1 Allison Manufacturing Sales Budget For The Quarter I Ended March 31 First QuarterSultanz Farkhan SukmanaNo ratings yet