Professional Documents
Culture Documents
Ice Ice.2.0
Uploaded by
ShanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ice Ice.2.0
Uploaded by
ShanCopyright:
Available Formats
Project:
mini ice maker
Simple Layout Of Processes:
Ice Manufacturing Plant
Salinity
Salinity is the measure of the number of grams of salts per kilogram of seawater, which is expressed in
parts per thousand. Parts per thousand can be defined as how many parts, or grams, of salt there are
per thousand parts, or kilogram (1,000 g), of seawater. The symbol for parts per thousand is ‰. The
symbol for parts per thousand (‰) is similar to the symbol for percent (%), which is parts per hundred,
but with an extra zero in the denominator. Parts per thousand is commonly abbreviated as ppt.
What is the freezing temperature of sea water?
The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes at about
−2 °C (28 °F). The freezing point of seawater decreases as salt concentration increases.
In solids/liquids the faster way of heat transfer is through
conduction than convection.
Using brine solution for making ice
Brine is used as a secondary fluid in large refrigeration installations for the transport of thermal
energy. Most commonly used brines are based on inexpensive calcium chloride and sodium chloride. It
is used because the addition of salt to water lowers the freezing temperature of the solution and the heat
transport efficiency can be greatly enhanced for the comparatively low cost of the material. The lowest
freezing point obtainable for NaCl brine is −21.1 °C (−6.0 °F) at the concentration of 23.3% NaCl by
weight.
Block ice is produced by placing water in galvanized iron cans or molds that are immersed in a
secondary coolant, brine (calcium or sodium chloride), which is kept cold by refrigerant (often ammonia)
expansion coils.
Actual experiment result on ice making process with the consideration of percent concentration
of brine solution.
According to their experiment, the lowest temperature to produce thick ice is -6.25 degree Celsius.
Popsicle mold vs metal mold
Ice was formed:
for Popsicle mold: 90 mins
for Metal mold: 110 mins
They have concluded that one factors why in popsicle
mold, water solidifies a little faster than in metal mold
was its surface area. The greater the surface area, the
faster the solidification.
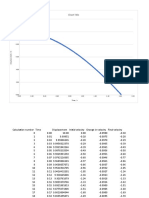
SODIUM CHLORIDE BRINE TABLES FOR 60° F (15.5° C)
BRINE STRENGTH POUND/GALLON BRINE GRAM/LITER BRINE *FREEZING POINT
Salometer Specific Baume NaCl %
NaCl Water NaCl Water °F °C
Degree Gravity Degree Wt.
0 1.000 0.0 0.00 0.000 8.328 0.0 998 +32.0 0
2 1.004 0.6 0.53 0.044 8.318 5.3 996 +31.5 -0.2
4 1.007 1.1 1.06 0.089 8.297 10.6 995 +31.1 -0.5
6 1.011 1.6 1.58 0.133 8.287 16.0 993 +30.5 -0.8
8 1.015 2.1 2.11 0.178 8.275 21.4 991 +30.0 -1.1
10 1.019 2.7 2.64 0.224 8.262 26.8 990 +29.3 -1.5
12 1.023 3.3 3.17 0.270 8.250 32.3 988 +28.8 -1.8
14 1.026 3.7 3.70 0.316 8.229 37.9 986 +28.2 -2.1
16 1.030 4.2 4.22 0.362 8.216 43.4 985 +27.6 -2.4
18 1.034 4.8 4.75 0.409 8.202 49.0 983 +27.0 -2.8
20 1.038 5.3 5.28 0.456 8.188 54.6 981 +26.4 -3.1
22 1.042 5.8 5.81 0.503 8.175 60.3 979 +25.7 -3.5
24 1.046 6.4 6.34 0.552 8.159 66.1 977 +25.1 -3.8
26 1.050 6.9 6.86 0.600 8.144 71.9 976 +24.4 -4.2
28 1.054 7.4 7.39 0.649 8.129 77.7 974 +23.7 -4.6
30 1.058 7.9 7.92 0.698 8.113 83.6 972 +23.0 -5.0
32 1.062 8.5 8.45 0.747 8.097 89.5 970 +22.3 -5.4
34 1.066 9.0 8.97 0.797 8.081 95.4 968 +21.6 -5.8
36 1.070 9.5 9.50 0.847 8.064 101.4 966 +20.9 -6.2
38 1.074 10.0 10.03 0.897 8.047 107.5 964 +20.2 -6.5
40 1.078 10.5 10.56 0.948 8.030 113.5 962 +19.4 -7.0
42 1.082 11.0 11.09 0.999 8.012 119.6 960 +18.7 -7.4
44 1.086 11.5 11.61 1.050 7.994 125.8 957 +17.9 -7.8
46 1.090 12.0 12.14 1.102 7.976 132.0 955 +17.1 -8.3
48 1.094 12.5 12.67 1.154 7.957 138.2 953 +16.2 -8.8
50 1.098 12.9 13.20 1.207 7.937 144.5 951 +15.4 -9.2
52 1.102 13.4 13.73 1.260 7.918 150.9 949 +14.5 -9.7
54 1.106 13.9 14.25 1.313 7.898 157.2 946 +13.7 -10.2
56 1.110 14.4 14.78 1.366 7.878 163.7 944 +12.8 -10.7
58 1.114 14.8 15.31 1.420 7.858 170.1 941 +11.8 -11.2
60 1.118 15.3 15.84 1.475 7.836 176.7 939 +10.9 -11.7
62 1.122 15.8 16.37 1.529 7.815 183.2 936 +9.9 -12.3
64 1.126 16.2 16.89 1.584 7.794 189.8 934 +8.9 -12.8
66 1.130 16.7 17.42 1.639 7.772 196.5 932 +7.9 -13.4
68 1.135 17.2 17.95 1.697 7.755 203.7 929 +6.8 -14.0
70 1.139 17.7 18.48 1.753 7.733 210.0 926 +5.7 -14.6
72 1.143 18.1 19.00 1.809 7.710 216.7 924 +4.6 -15.2
74 1.147 18.6 19.53 1.866 7.686 223.5 921 +3.4 -15.9
76 1.152 19.1 20.06 1.925 7.669 230.6 918 +2.2 -16.5
78 1.156 19.6 20.59 1.982 7.645 237.4 916 +1.0 -17.2
80 1.160 20.0 21.12 2.040 7.620 244.4 913 -0.4 -18.0
82 1.164 20.4 21.64 2.098 7.596 251.5 911 -1.6 -18.6
84 1.169 21.0 22.17 2.158 7.577 258.5 908 -3.0 -19.4
86 1.173 21.4 22.70 2.218 7.551 265.7 905 -4.4 -20.2
88 1.178 21.9 23.23 2.279 7.531 272.9 902 -5.8 -21.0
**88.3 1.179 22.0 23.31 2.288 7.528 274.1 901 **-6.0 **-21.0
90 1.182 22.3 23.75 2.338 7.506 280.1 899 -1.1 -18.5
92 1.186 22.7 24.28 2.398 7.479 287.4 896 +4.8 -15.0
94 1.191 23.3 24.81 2.459 7.460 294.7 893 +11.1 -11.6
95 1.193 23.5 25.08 2.491 7.444 298.4 892 +14.4 -9.8
96 1.195 23.7 25.34 2.522 7.430 302.1 890 +18.0 -7.8
97 1.197 23.9 25.60 2.552 7.417 305.8 888 +21.6 -5.8
98 1.200 24.2 25.87 2.585 7.409 309.6 887 +25.5 -3.6
99 1.202 24.4 26.13 2.616 7.394 313.4 886 +29.8 -1.2
99.6 1.203 24.5 26.29 2.634 7.386 315.6 885 ***+32.3 ***+0.2
100 1.204 24.6 26.40 2.647 7.380 317.2 884 ****+60.0 ****+15.5
The above table applies to brine tested at the temperature of 60° F.
For brine tested at a warmer or colder temperature than 60° F. see Table of Salometer Corrections on the next page.
* Temperature at which freezing begins. Ice forms, brine concentrates, and the freezing point lowers to eutectic.
** Eutectic point. For brines stronger than eutectic, the temperatures shown are the saturation temperatures for sodium chloride dihydrate.
Brines stronger than eutectic deposit excess sodium chloride as dihydrate when cooled, and freeze at eutectic.
*** Transition temperature from anhydrous salt to dihydrate.
**** Saturated brine at 60° F.
You might also like
- Sodium Chloride TableDocument1 pageSodium Chloride TableKC BALINo ratings yet
- TF-8695 Therminol-66 Technical Bulletin-4Document1 pageTF-8695 Therminol-66 Technical Bulletin-4Graphios UtaNo ratings yet
- Steam Table From PerryDocument2 pagesSteam Table From PerryIndrawNo ratings yet
- Table A.23 Physical Properties of Selected Fluids (Engineering Thermodynamics by Burghardt & Harbach)Document1 pageTable A.23 Physical Properties of Selected Fluids (Engineering Thermodynamics by Burghardt & Harbach)lemuel andrezaNo ratings yet
- R134a Sobrecalentado MEC 2254 DOCENTE: EDGAR S. PEÑARANDA M. UTO FNI Pag1 P 0.6 Bar P 1barDocument3 pagesR134a Sobrecalentado MEC 2254 DOCENTE: EDGAR S. PEÑARANDA M. UTO FNI Pag1 P 0.6 Bar P 1barSelena ZepitaNo ratings yet
- Rainfall-Log Pierson Type IIIDocument4 pagesRainfall-Log Pierson Type IIIisnanhidayNo ratings yet
- Appendix C Steam TablesDocument8 pagesAppendix C Steam TablesWin Alfalah Nasution100% (1)
- Calculation of Curve Widening For The Alternative DesignDocument13 pagesCalculation of Curve Widening For The Alternative DesignDaylath MendisNo ratings yet
- App3Document2 pagesApp3hamidrezaee008No ratings yet
- Craciun Daniel Andrei Mecanisme Cama-TachetDocument3 pagesCraciun Daniel Andrei Mecanisme Cama-TachetDragonManYTB 1No ratings yet
- Gravity 1 (Without F)Document272 pagesGravity 1 (Without F)Dhairya GandhiNo ratings yet
- Tabel A.5. Untuk Gas Pada Saturated LiquidDocument3 pagesTabel A.5. Untuk Gas Pada Saturated LiquidAsnifNo ratings yet
- Gravity 1 (With F)Document273 pagesGravity 1 (With F)Dhairya GandhiNo ratings yet
- Density and Specific Weight of Air at 1 Atmosphere PressureDocument2 pagesDensity and Specific Weight of Air at 1 Atmosphere Pressurehaha 223No ratings yet
- B-05 Standard Roll Groove-RevGDocument2 pagesB-05 Standard Roll Groove-RevGDeniNo ratings yet
- UPVC Drainage Pipes & Fittings - ACDocument101 pagesUPVC Drainage Pipes & Fittings - ACsushant_moreyNo ratings yet
- Tablas Termodinamicas Amoniaco@Claus Borgnakke, Richard E. Sonntag (7th Edition)Document6 pagesTablas Termodinamicas Amoniaco@Claus Borgnakke, Richard E. Sonntag (7th Edition)Olman VargasNo ratings yet
- AMMONIA and R134a (Satd and SH) (English and SI)Document24 pagesAMMONIA and R134a (Satd and SH) (English and SI)MinjdeDios0% (1)
- Refrigerants Table-SI Unit PDFDocument31 pagesRefrigerants Table-SI Unit PDFAdriel JohnNo ratings yet
- 1.6 2017 ASHRAE Handbook-Fundamentals (SI) : Table 3 Thermodynamic Properties of Water at Saturation (Continued)Document1 page1.6 2017 ASHRAE Handbook-Fundamentals (SI) : Table 3 Thermodynamic Properties of Water at Saturation (Continued)metroroadNo ratings yet
- GRAFICADocument16 pagesGRAFICALENNI DAYARA HERNANDEZ GARCIANo ratings yet
- Ministry of Physical Infrastructure Development OfficeDocument2 pagesMinistry of Physical Infrastructure Development OfficeCivil EngineeringNo ratings yet
- Section 12-2 PDFDocument10 pagesSection 12-2 PDFMohamed AbozeimaNo ratings yet
- Physical Properties of Liquid Water: Waterproperties - Doc Page 1 of 5Document5 pagesPhysical Properties of Liquid Water: Waterproperties - Doc Page 1 of 5ghulammohyuddinNo ratings yet
- B-05 Standard Roll Groove-RevFDocument2 pagesB-05 Standard Roll Groove-RevFAgreen AnggadaNo ratings yet
- Diagram AsDocument11 pagesDiagram AsJhon MagañoNo ratings yet
- Friction Loss Water in Feet Per 100 FT of PipeDocument20 pagesFriction Loss Water in Feet Per 100 FT of PipeME_engineerNo ratings yet
- SM II - Global WindWind Load On Main ModelDocument31 pagesSM II - Global WindWind Load On Main ModelsundarNo ratings yet
- Tramo de Apertura PasesDocument7 pagesTramo de Apertura PasesDavid ToroNo ratings yet
- Planillas Topografica HoyDocument7 pagesPlanillas Topografica Hoysaul felipe junes floresNo ratings yet
- 920-920R NR SizingDocument2 pages920-920R NR SizingKonrad SzwedoNo ratings yet
- QNS DataDocument5 pagesQNS DataAryamaan SinghNo ratings yet
- NSSC Process Optimization: Ii. Spent Liquors: AbstractDocument8 pagesNSSC Process Optimization: Ii. Spent Liquors: AbstractKarteek KandalaNo ratings yet
- VOLUMENDocument8 pagesVOLUMENnelson molianes doriaNo ratings yet
- R134a SuperDocument6 pagesR134a Superprem singhNo ratings yet
- 32 - Nguyễn Ngọc Trân - T10Document7 pages32 - Nguyễn Ngọc Trân - T10Ngọc TrânNo ratings yet
- MWH S Water Treatment Principles and Design Third Edition - 2012 - Crittenden - Appendix C Physical Properties of WaterDocument2 pagesMWH S Water Treatment Principles and Design Third Edition - 2012 - Crittenden - Appendix C Physical Properties of WaterbastianpurwaNo ratings yet
- HPV Hydrostatics Run2Document6 pagesHPV Hydrostatics Run2maaathanNo ratings yet
- Tx69299app PDFDocument9 pagesTx69299app PDFZachariah JosephNo ratings yet
- P4 (X) x4-1,1x3+2,3x2+0,5x+3,3 0 Metode Bairstow: Nama: Rahmad Denny Aulia Nim: 16521241Document5 pagesP4 (X) x4-1,1x3+2,3x2+0,5x+3,3 0 Metode Bairstow: Nama: Rahmad Denny Aulia Nim: 16521241Tegar gayuh pambudhiNo ratings yet
- P4 (X) x4-1,1x3+2,3x2+0,5x+3,3 0 Metode Bairstow: Nama: Rahmad Denny Aulia Nim: 16521241Document5 pagesP4 (X) x4-1,1x3+2,3x2+0,5x+3,3 0 Metode Bairstow: Nama: Rahmad Denny Aulia Nim: 16521241Tegar gayuh pambudhiNo ratings yet
- Ef400413r Si 001Document5 pagesEf400413r Si 001ravenNo ratings yet
- Data Lingkungan - RevDocument137 pagesData Lingkungan - RevGallend SNo ratings yet
- R 134a PDFDocument1 pageR 134a PDFFabian de Jesus Orozco MartinezNo ratings yet
- New Microsoft PowerPoint Presentation (1) (تم حفظه تلقائيا) PDFDocument26 pagesNew Microsoft PowerPoint Presentation (1) (تم حفظه تلقائيا) PDF123No ratings yet
- Datasheet 6x19 Fibre Core GalvanisedDocument1 pageDatasheet 6x19 Fibre Core GalvanisedKurnia Adi WibowoNo ratings yet
- Tablas Transferencia de MasaDocument25 pagesTablas Transferencia de MasaEdwin GuillénNo ratings yet
- Physical Properties of Gases and Liquids: AppendixDocument13 pagesPhysical Properties of Gases and Liquids: AppendixMarcos Vinicius KonopkaNo ratings yet
- Ejercicio Van - TirDocument8 pagesEjercicio Van - Tirluis alberto quispe vasquezNo ratings yet
- Sheet Size ChartDocument7 pagesSheet Size ChartTusharNo ratings yet
- Formate Brine TableDocument12 pagesFormate Brine Tables v poyil100% (1)
- Saturated Water and Steam (Pressure-Based Table)Document1 pageSaturated Water and Steam (Pressure-Based Table)Brandon brownNo ratings yet
- Column Design ExampleDocument5 pagesColumn Design ExampleBert NiebresNo ratings yet
- Motorola Six Sigma Conversion TableDocument1 pageMotorola Six Sigma Conversion TableMars HNo ratings yet
- NEC - Table 8 Conductor PropertiesDocument1 pageNEC - Table 8 Conductor PropertiesRogelio Revetti25% (4)
- Fecto Sugar: 2009 2008 Liquidity RatioDocument10 pagesFecto Sugar: 2009 2008 Liquidity RatioMushal JamilNo ratings yet
- Aceros No. As (cm2) FC' (kgf/cm2) Fy (kgf/cm2) B (CM) Cuantia Min H (CM) Cuantia Max D (CM) Rec (CM) Fi Estribo M Refuerzo InfDocument13 pagesAceros No. As (cm2) FC' (kgf/cm2) Fy (kgf/cm2) B (CM) Cuantia Min H (CM) Cuantia Max D (CM) Rec (CM) Fi Estribo M Refuerzo InfAlejandro SaenzNo ratings yet
- United States Census Figures Back to 1630From EverandUnited States Census Figures Back to 1630No ratings yet
- Government Publications: Key PapersFrom EverandGovernment Publications: Key PapersBernard M. FryNo ratings yet
- Math Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesFrom EverandMath Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesRating: 5 out of 5 stars5/5 (3)
- Tesda RTC VII Risk Management Plan 2022Document7 pagesTesda RTC VII Risk Management Plan 2022ShanNo ratings yet
- Accomplishment Sep2022 LastDocument1 pageAccomplishment Sep2022 LastShanNo ratings yet
- DCL Individual Output - Self AssessmentDocument2 pagesDCL Individual Output - Self AssessmentShanNo ratings yet
- Electronic-Devices Mod2Document21 pagesElectronic-Devices Mod2ShanNo ratings yet
- Annex C - Form 1 - STAR Summary MACHINING NC IIDocument10 pagesAnnex C - Form 1 - STAR Summary MACHINING NC IIShanNo ratings yet
- TESDA Circular No. 143-2020Document36 pagesTESDA Circular No. 143-2020ShanNo ratings yet
- International Journal of Emerging Trends in Engineering ResearchDocument8 pagesInternational Journal of Emerging Trends in Engineering ResearchShanNo ratings yet
- ICT Conference 2022-83Document1 pageICT Conference 2022-83ShanNo ratings yet
- CCAP 2022 Philipines Application Seminar - 052422Document6 pagesCCAP 2022 Philipines Application Seminar - 052422ShanNo ratings yet
- Maintenance Job Order-Converted (ELECTRICAL)Document4 pagesMaintenance Job Order-Converted (ELECTRICAL)ShanNo ratings yet
- CBLM Conduct Training Need Analysis Ver. 0Document110 pagesCBLM Conduct Training Need Analysis Ver. 0ShanNo ratings yet
- Ice Ice.2.0Document4 pagesIce Ice.2.0ShanNo ratings yet
- 2019 Star-Instrument - Machining NC IIDocument51 pages2019 Star-Instrument - Machining NC IIShanNo ratings yet
- Cy 2020Document1,270 pagesCy 2020ShanNo ratings yet
- Sources of IncomeDocument1 pageSources of IncomeShanNo ratings yet
- Rme Problem Set No. 1Document5 pagesRme Problem Set No. 1ShanNo ratings yet
- TVET Brief Issue No. 4 - CBT and Capacity-Based TrainingDocument7 pagesTVET Brief Issue No. 4 - CBT and Capacity-Based TrainingShanNo ratings yet
- With Increasing Temperature, Salinity Decreases.... and Conversely, With Decreasing Temperature, Salinity Increases. You Ask Why..... Density!Document1 pageWith Increasing Temperature, Salinity Decreases.... and Conversely, With Decreasing Temperature, Salinity Increases. You Ask Why..... Density!ShanNo ratings yet
- With Increasing Temperature, Salinity Decreases.... and Conversely, With Decreasing Temperature, Salinity Increases. You Ask Why..... Density!Document1 pageWith Increasing Temperature, Salinity Decreases.... and Conversely, With Decreasing Temperature, Salinity Increases. You Ask Why..... Density!ShanNo ratings yet
- Unsafe and Unhealthy ActsDocument29 pagesUnsafe and Unhealthy ActsShanNo ratings yet
- MANUAL PIPE BEVELLING MACHINE (Revised 2021)Document10 pagesMANUAL PIPE BEVELLING MACHINE (Revised 2021)ShanNo ratings yet
- Engineering Ex Perv 00000 I 00254Document76 pagesEngineering Ex Perv 00000 I 00254ShanNo ratings yet
- Tesda MVGDocument2 pagesTesda MVGShanNo ratings yet
- PecDocument5 pagesPecvon11No ratings yet
- The Use of Ice On Board Smaller Fishing Vessels Is IncreasingDocument2 pagesThe Use of Ice On Board Smaller Fishing Vessels Is IncreasingShanNo ratings yet
- Matheng Skript 1213Document227 pagesMatheng Skript 1213mcrajpuraNo ratings yet
- Sept 2019Document6 pagesSept 2019ShanNo ratings yet
- Freon 404A - Thermodynamic Properties-Si PDFDocument28 pagesFreon 404A - Thermodynamic Properties-Si PDFFaustoNo ratings yet
- Sept 2019Document6 pagesSept 2019ShanNo ratings yet
- Mgo Los Banos Laguna-Community Affairs Officer IIDocument1 pageMgo Los Banos Laguna-Community Affairs Officer IICESHNo ratings yet
- JERES-J-607 Burner Management Systems For SRU TrainsDocument24 pagesJERES-J-607 Burner Management Systems For SRU TrainsMahi IndraNo ratings yet
- HDM Method PDFDocument117 pagesHDM Method PDFBonagiri DheerajNo ratings yet
- 1.4435 - C Stainless Steel DetailsDocument3 pages1.4435 - C Stainless Steel DetailsmeenakshiNo ratings yet
- Unit 8. New Ways To Learn: Part I. PhoneticsDocument14 pagesUnit 8. New Ways To Learn: Part I. PhoneticsPhạm Gia LợiNo ratings yet
- Dictionaries Godot GDScript Tutorial Ep 12 Godot TutorialsDocument4 pagesDictionaries Godot GDScript Tutorial Ep 12 Godot TutorialsChris LewinskyNo ratings yet
- General Information NC Detector: SignatureDocument2 pagesGeneral Information NC Detector: SignatureEvelin FriasNo ratings yet
- MS Disc Brake CaliperDocument2 pagesMS Disc Brake Caliperghgh140No ratings yet
- TW Ebook Product Thinking Playbook Digital2pdfDocument172 pagesTW Ebook Product Thinking Playbook Digital2pdfgermtsNo ratings yet
- Ps10ex Pressure Switch PresostatoDocument1 pagePs10ex Pressure Switch PresostatoJULIO AREVALONo ratings yet
- Embase - Other - EmbaseScopusOverview Base de DatosDocument5 pagesEmbase - Other - EmbaseScopusOverview Base de DatosAditya GhoshNo ratings yet
- Postgresql 11 A4 PDFDocument2,621 pagesPostgresql 11 A4 PDFGiuliano PertileNo ratings yet
- Tech M Service LetterDocument1 pageTech M Service LetterRahul upadhyayNo ratings yet
- Intrusion Detection System IDS Seminar ReportDocument18 pagesIntrusion Detection System IDS Seminar ReportSahil SethiNo ratings yet
- Marketing Manager: at Aztec GroupDocument2 pagesMarketing Manager: at Aztec GroupAgnish GhatakNo ratings yet
- Computer Vision - Ipynb - ColaboratoryDocument17 pagesComputer Vision - Ipynb - Colaboratoryzb laiNo ratings yet
- Sample Midterm (Lab)Document3 pagesSample Midterm (Lab)Shahab designerNo ratings yet
- The Environment and Corporate Culture: True/False QuestionsDocument21 pagesThe Environment and Corporate Culture: True/False QuestionsĐỗ Hiếu ThuậnNo ratings yet
- Huawei FusionServer RH8100 V3 Data SheetDocument4 pagesHuawei FusionServer RH8100 V3 Data Sheetpramod BhattNo ratings yet
- Submittal Patterson Bombs PDFDocument77 pagesSubmittal Patterson Bombs PDFFred GarciaNo ratings yet
- Perilaku Masyarakat Terhadap Kesehatan Lingkungan (Studi Di Pantai Desa Ketong Kecamatan Balaesang Tanjung Kabupaten Donggala)Document11 pagesPerilaku Masyarakat Terhadap Kesehatan Lingkungan (Studi Di Pantai Desa Ketong Kecamatan Balaesang Tanjung Kabupaten Donggala)Ali BaktiNo ratings yet
- Analytics-Based Investigation & Automated Response With AWS + Splunk Security SolutionsDocument37 pagesAnalytics-Based Investigation & Automated Response With AWS + Splunk Security SolutionsWesly SibagariangNo ratings yet
- ProjectDocument14 pagesProjectNavneet singh50% (2)
- SABA Migration RunBook v1.0 20210913xlsxDocument54 pagesSABA Migration RunBook v1.0 20210913xlsxZakaria AlmamariNo ratings yet
- Psychology of Color2Document33 pagesPsychology of Color2abdikani abdilaahiNo ratings yet
- NAVEEN Final Project 2Document83 pagesNAVEEN Final Project 2syed bismillahNo ratings yet
- Meridium Enterprise APM ModulesAndFeaturesDeploymentDocument517 pagesMeridium Enterprise APM ModulesAndFeaturesDeploymenthellypurwantoNo ratings yet
- Ab 521 Requirements For Engineered Pressure EnclosuresDocument39 pagesAb 521 Requirements For Engineered Pressure EnclosuresCarlos Maldonado SalazarNo ratings yet
- EM - I-Assignment - II (NA) - 1Document2 pagesEM - I-Assignment - II (NA) - 1deepakNo ratings yet
- Section - : Exit To Main MenuDocument186 pagesSection - : Exit To Main Menuadi67% (3)