100% found this document useful (1 vote)
1K views9 pagesPQ-Sample FOUR
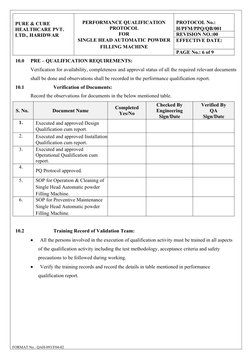
This document provides a performance qualification protocol for a single head automatic powder filling machine located in the ointment section of Q Block at Pure & Cure Healthcare Pvt. Ltd. in Haridwar, India. The protocol outlines objectives to qualify the equipment as suitable for its intended use and to produce products meeting predefined acceptance criteria. It describes the equipment, evaluation tests and checks to be performed, responsibilities of involved departments, and frequency of requalification. The goal of following this protocol is to verify through documented tests that the powder filling machine performs as intended.
Uploaded by
cpkakopeCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOC, PDF, TXT or read online on Scribd
100% found this document useful (1 vote)
1K views9 pagesPQ-Sample FOUR
This document provides a performance qualification protocol for a single head automatic powder filling machine located in the ointment section of Q Block at Pure & Cure Healthcare Pvt. Ltd. in Haridwar, India. The protocol outlines objectives to qualify the equipment as suitable for its intended use and to produce products meeting predefined acceptance criteria. It describes the equipment, evaluation tests and checks to be performed, responsibilities of involved departments, and frequency of requalification. The goal of following this protocol is to verify through documented tests that the powder filling machine performs as intended.
Uploaded by
cpkakopeCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOC, PDF, TXT or read online on Scribd