Professional Documents
Culture Documents
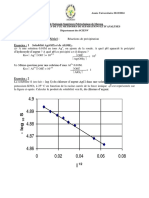
Equilibria Analytical Chemistry Solubility: Ionic IN II
Uploaded by
Chadrekzy January PungosOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Equilibria Analytical Chemistry Solubility: Ionic IN II
Uploaded by
Chadrekzy January PungosCopyright:
Available Formats
300 IONIC EQUILIBRIA IN ANALYTICAL CHEMISTRY
Table II — Continued
c
Solubility ProductConstants at 25
Sub stance Formula sp
Calcium hydroxide Ca(0H)2 5.5 x 10"
Calcium iodate 7.0 x 10
Ca(l03)2
Calcium oxalate 2.1 x 10
CaCgO^
.2?
Calcium phosphate 2.0 x 10
Calcium sulfate 2.k x 10
CaSOj^
-29
Cerium(lll) oxalate 2.5 x 10*
Ce2(C204)3 •11
Cerium(lll) sulfide Ce S 6.0 x 10
2 3
-21
Chromium arsenate CrAsO. 7.8 x 10
•31
Chromium hydroxide Cr(OH) 7.0 x 10
.13
Cobalt carbonate CoCO .0 x 10
-16
Cobalt hydroxide 2.0 x 10
Co(0H)2
-8
Cobalt oxalate CoC 0, k.O x 10
2 h
'23
Cobalt sulfide CoS 8.0 x 10'
Copper(l) bromide CuBr 5.3 x 10"
Copper(l) chloride CuCl 3-2 x 10"
-11
Copper (i) cyanide CuCN 1.0 x 10
-12
Copper(l) iodide Cul 1.1 x 10
he
Copper(l) sulfide 1.0 x 10
Cu2S
Copper(l) thiocyanate CuNCS 1.6 x 10"11
-10
Copper(ll) carbonate CuCO 2.5 x 10
-6
Copper ( II) chromate CuCrO. 3.6 x 10
-20
Copper(ll) hydroxide Cu(OH), 2.2 x 10
C
-36
Copper(ll) sulfide CuS 8.0 x 10
-11
Iron(ll) carbonate FeCO.
3
3-5 x 10
.16
Iron(ll) hydroxide Fe(OH), 8.0 x 10'
-7
Iron(ll) oxalate
FeC204
2.0 x 10
You might also like
- Instrumental Chemistry For Engineers (CHE515) : Prepared byDocument31 pagesInstrumental Chemistry For Engineers (CHE515) : Prepared byJohan Aliff100% (1)
- Slag Defects in Grey IronDocument1 pageSlag Defects in Grey Ironarnaldorcr8646100% (1)
- Science Form 3 July TestDocument7 pagesScience Form 3 July TestNorafiza Hashim100% (1)
- Ility: Appendix IIDocument1 pageIlity: Appendix IIChadrekzy January PungosNo ratings yet
- Solubility ProductsDocument2 pagesSolubility Productsgracemizzi6No ratings yet
- CHEM 18 4th Exam Problem Set (2019) PDFDocument4 pagesCHEM 18 4th Exam Problem Set (2019) PDFElton BoholstNo ratings yet
- Heavy Metals Removal - WastewaterDocument43 pagesHeavy Metals Removal - WastewaterSong Nguyen NguyenNo ratings yet
- Electrical Double Layer Cu20Document14 pagesElectrical Double Layer Cu20Anonymous PT1b9IWNo ratings yet
- Table Constanta of SolubilitiesDocument6 pagesTable Constanta of SolubilitiesuhsduNo ratings yet
- Science PortfolioDocument20 pagesScience PortfolioAkshitaNo ratings yet
- SG Minerals ChartDocument2 pagesSG Minerals ChartMikeWalsheNo ratings yet
- Tab 6 F Sandstone Acidizing Chem and Design PDF FreeDocument34 pagesTab 6 F Sandstone Acidizing Chem and Design PDF FreeHelyaNo ratings yet
- Jasinski 1987Document5 pagesJasinski 1987Het DedhiaNo ratings yet
- RedoxDocument15 pagesRedoxInês AlmeidaNo ratings yet
- S&P Block PDFDocument1 pageS&P Block PDFvrtbhgmngfNo ratings yet
- Hardness of Minerals and Ceramics Reference: Material Formula Mohs Modified Mohs KnoopDocument1 pageHardness of Minerals and Ceramics Reference: Material Formula Mohs Modified Mohs KnoopantonioNo ratings yet
- Aqueous Solution Chemistry-ExercisesDocument7 pagesAqueous Solution Chemistry-ExercisesragustochemNo ratings yet
- Adobe Scan Feb 04, 2024Document1 pageAdobe Scan Feb 04, 2024Pushkar KumarNo ratings yet
- PCM Chapter 01 Part BDocument7 pagesPCM Chapter 01 Part BAlif AzmirNo ratings yet
- Metal Recovery From Waste SludgesDocument7 pagesMetal Recovery From Waste SludgesHugo WizenbergNo ratings yet
- Tackling Impurities in Copper ConcentratesDocument10 pagesTackling Impurities in Copper ConcentratesEduardo CandelaNo ratings yet
- CMP2015 - Challenges in Niobium FlotationDocument11 pagesCMP2015 - Challenges in Niobium FlotationrodrigoNo ratings yet
- Part 4 - Manufacturing Sodium Carbonate and The Solvay ProcessDocument4 pagesPart 4 - Manufacturing Sodium Carbonate and The Solvay Processracha rachaNo ratings yet
- Unit 08b: Advanced Hydrogeology: Groundwater ChemistryDocument22 pagesUnit 08b: Advanced Hydrogeology: Groundwater ChemistryWellfroNo ratings yet
- MetallurgyDocument6 pagesMetallurgyArpit SharmaNo ratings yet
- Select: Kvpy - SX - Metallurgy # 652Document11 pagesSelect: Kvpy - SX - Metallurgy # 652Jatindra PatelNo ratings yet
- Localized CorrosionDocument11 pagesLocalized CorrosionJack AndreasNo ratings yet
- 03-General Principals of Metallurgical operations-Sol.-Final-EDocument3 pages03-General Principals of Metallurgical operations-Sol.-Final-EAbhishek RavirajNo ratings yet
- 76 Direct Hydrogenation of Aliphatic Carboxylic Acids To Corresponding Aldehydes With Cr203 CatalystDocument4 pages76 Direct Hydrogenation of Aliphatic Carboxylic Acids To Corresponding Aldehydes With Cr203 Catalystrommy agurto palaciosNo ratings yet
- Worth ReadingDocument4 pagesWorth ReadingPassmore DubeNo ratings yet
- Extraction of Copper: (From Sulphide Ores)Document25 pagesExtraction of Copper: (From Sulphide Ores)Abhishek KumarNo ratings yet
- Metallurgy Short NotesDocument8 pagesMetallurgy Short NotesTerabaap AayaNo ratings yet
- HO / "' OH OH: Li SoDocument4 pagesHO / "' OH OH: Li So葉建豪No ratings yet
- Trade Effluent Discharge LimitsDocument2 pagesTrade Effluent Discharge Limitsjiaolei9848No ratings yet
- RAM and RFF QuestionsDocument2 pagesRAM and RFF QuestionsMariam EissaNo ratings yet
- A Periodic TableDocument1 pageA Periodic Tabletownsenr94No ratings yet
- Ores & Metllurgy: Chapter Practice ProblemsDocument3 pagesOres & Metllurgy: Chapter Practice Problemsyashik goyalNo ratings yet
- Short Notes Chapter 6Document2 pagesShort Notes Chapter 6alinNo ratings yet
- NBS 25-10 Картки Стандарт Зразкiв Рентген-дифракцii 1972Document166 pagesNBS 25-10 Картки Стандарт Зразкiв Рентген-дифракцii 1972CementarNo ratings yet
- Nomenclature ReviewDocument1 pageNomenclature ReviewGabriel ParksNo ratings yet
- Chloride Metallurgy - Process Technology Development - : Edgar PeekDocument39 pagesChloride Metallurgy - Process Technology Development - : Edgar PeekMauricioTeranAguilarNo ratings yet
- Heat Cao S Ho Ca Oh Aq: Hol H G OgDocument12 pagesHeat Cao S Ho Ca Oh Aq: Hol H G OgValyanaNo ratings yet
- M Topics Chemistry TP 10.1 - 10.6Document258 pagesM Topics Chemistry TP 10.1 - 10.6hataf bayarNo ratings yet
- Catch Up Plan Aras RendahDocument12 pagesCatch Up Plan Aras RendahNurnadia IzzatieNo ratings yet
- Basic Principle of Extraction - DTS 1 Adv (Archive) SolDocument2 pagesBasic Principle of Extraction - DTS 1 Adv (Archive) SolGeeta KharbNo ratings yet
- LMBNG Unsr, TTK DDH, LLHDocument3 pagesLMBNG Unsr, TTK DDH, LLHRoselinaNo ratings yet
- MetallurgyDocument39 pagesMetallurgyPrabhakar BandaruNo ratings yet
- Pre-AP Chem Equation Sheet and Periodic TableDocument3 pagesPre-AP Chem Equation Sheet and Periodic Tablemeghan.kennedyNo ratings yet
- 12C 29 Extractive Metallurgy PDFDocument63 pages12C 29 Extractive Metallurgy PDFgovind_galamNo ratings yet
- Concentrate Copper Waste Treatment: Samsung Semiconductor Austin EmewcorporationDocument24 pagesConcentrate Copper Waste Treatment: Samsung Semiconductor Austin EmewcorporationJOSE MACASSINo ratings yet
- Metallurgy Theory PDFDocument17 pagesMetallurgy Theory PDFPrajwal TalwalkarNo ratings yet
- List of Common Gangue Minerals - Introduction To Mineral ExplorationDocument1 pageList of Common Gangue Minerals - Introduction To Mineral ExplorationKareemAmen100% (1)
- Treating Metal Finishing Wastewater PDFDocument7 pagesTreating Metal Finishing Wastewater PDFSong Nguyen NguyenNo ratings yet
- TD N°1Document2 pagesTD N°1Native Emerick Kokea TielaNo ratings yet
- S Block MTG PyqDocument6 pagesS Block MTG PyqAJAD YADAVNo ratings yet
- Ionic Radius - Wikipedia PDFDocument29 pagesIonic Radius - Wikipedia PDFடேவிட் ஸ்No ratings yet
- S&P Block PDFDocument1 pageS&P Block PDFvrtbhgmngfNo ratings yet
- Chapter 10 Lead PDFDocument12 pagesChapter 10 Lead PDFjessy eghNo ratings yet
- MetallurgyDocument26 pagesMetallurgySitabai JadhavNo ratings yet
- NSS Chemistry Part 3 Metals - LQDocument25 pagesNSS Chemistry Part 3 Metals - LQミーチェルNo ratings yet
- Review, Evolution and Optimization of The Kansanshi Mixed Copper Ore TreatmentDocument12 pagesReview, Evolution and Optimization of The Kansanshi Mixed Copper Ore TreatmentTinashe BareNo ratings yet
- Structure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsFrom EverandStructure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsNo ratings yet
- Steel Classification, Types, Heat Treatment and ApplicationsDocument21 pagesSteel Classification, Types, Heat Treatment and ApplicationsVed VadakeNo ratings yet
- Carbon Monoxide Hydrogenation Over Metal Loaded AluminophosphatesDocument14 pagesCarbon Monoxide Hydrogenation Over Metal Loaded AluminophosphatesNeil MilestoneNo ratings yet
- Reduction Swelling of Iron OxidesDocument32 pagesReduction Swelling of Iron OxidesMuykundan MenonNo ratings yet
- HyMelt Gasification Process Development ProjectDocument50 pagesHyMelt Gasification Process Development ProjectBá Văn TôNo ratings yet
- Control Del Aire Progrmacion LinealDocument8 pagesControl Del Aire Progrmacion LinealWilliam David Gil GaravitoNo ratings yet
- Kendriya Vidyalaya Sangathan: Chennai RegionDocument281 pagesKendriya Vidyalaya Sangathan: Chennai RegionAshray EapurNo ratings yet
- Stainless Steel Welded Pipes and Tubes For General Services - SpecificationDocument26 pagesStainless Steel Welded Pipes and Tubes For General Services - Specificationocsspectro100% (1)
- Chemistry Exam Term 3 EOT Form 4Document19 pagesChemistry Exam Term 3 EOT Form 4nisaa wilsonNo ratings yet
- GenChem AssignmentDocument12 pagesGenChem AssignmentGivenchy SanicoNo ratings yet
- CHM361 Result (EXP 2)Document4 pagesCHM361 Result (EXP 2)Hanis SyazwaniNo ratings yet
- Specification For Carbon Steel Electrodes For Shielded Metal Arc WeldingDocument42 pagesSpecification For Carbon Steel Electrodes For Shielded Metal Arc WeldingArmando Lujan VelazquezNo ratings yet
- Factors To Consider in Selecting A Dispersant For Treating Industrial Water SystemsDocument15 pagesFactors To Consider in Selecting A Dispersant For Treating Industrial Water SystemsmnasiroleslamiNo ratings yet
- Star Ocean Item CreationDocument8 pagesStar Ocean Item Creationn1ickLNo ratings yet
- Color AffectsDocument4 pagesColor Affectshyde2520015754No ratings yet
- Fugleberg SigmundDocument130 pagesFugleberg SigmundEdon BediNo ratings yet
- Foaming IndexDocument5 pagesFoaming Indexsaibal_silNo ratings yet
- Chemisrty Worksheets2Document3 pagesChemisrty Worksheets2Karthikeyan LakshmananNo ratings yet
- Module 2BDocument22 pagesModule 2BOluwasegun OkajareNo ratings yet
- Magnetic Effects in WeighingDocument3 pagesMagnetic Effects in WeighingiptNo ratings yet
- ASTM Comparison PDFDocument1 pageASTM Comparison PDFRaja HoneNo ratings yet
- Comparison Astm and JisDocument4 pagesComparison Astm and JisGokulAgNo ratings yet
- 9202 1 QP InternationalChemistry G 4nov21!07!00 GMTDocument32 pages9202 1 QP InternationalChemistry G 4nov21!07!00 GMTBob yuNo ratings yet
- My TestDocument20 pagesMy TestHidayah TeacherNo ratings yet
- VSA Type QuestionsDocument7 pagesVSA Type QuestionsTapas BanerjeeNo ratings yet
- Avk Universal Flange Adaptor PN 16 603Document2 pagesAvk Universal Flange Adaptor PN 16 603Sharon LambertNo ratings yet
- Separation TechniquesDocument5 pagesSeparation TechniquesSAMEERACH2009No ratings yet
- Ecw331 Chapter 10 FinalDocument34 pagesEcw331 Chapter 10 FinalNaqib KamarozamanNo ratings yet
- 10.1007@978 3 030 14918 5Document789 pages10.1007@978 3 030 14918 5Сергей ГубскийNo ratings yet