Professional Documents
Culture Documents
MIDTERMS
Uploaded by
MelindaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
MIDTERMS
Uploaded by
MelindaCopyright:
Available Formats
MIDTERMS
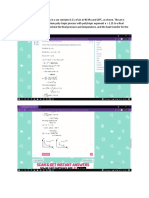
1. A piston–cylinder device contains 0.6 kg of steam at 200 oC and 0.5 MPa. Steam is cooled at
constant pressure until one-half of the mass condenses. Determine the volume change in m3.
2. A mass of 5 kg of saturated water vapor at 300 kPa is heated at constant pressure until the
temperature reaches 200 oC. Calculate the work done by the steam during this process in KJ.
3. A mixture with 70% quality at 500 kPa is heated isothermally until its pressure is 300 kPa. Find
the heat added during the process in KJ/Kg.
4. A mass of 200 g of saturated liquid water is completely vaporized at a constant pressure of 100
kPa. Determine (the amount of energy transferred to the water in KJ.
5. Superheated water vapor at 180 psia and 500 oF is allowed to cool at constant volume until the
temperature drops to 250 oF. At the final state, determine the enthalpy in Btu/lbm.
6. A piston-cylinder device initially contains 50 L of liquid water at 40 oC and 200 kPa. Heat is
transferred to the water at constant pressure until the entire liquid is vaporized Determine the
total enthalpy change in KJ.
7. A boiler receives a feedwater at 1200 psia and 250 oF and delivers a steam from the superheated
900 psia and 950 oF. Find the heat added in BTU/Lb
8. Steam at 2 MPa and 250°C in a rigid cylinder is cooled until the quality is 30%. Find the heat
rejected from the cylinder in KJ/Kg.
9. A piston-cylinder device initially contains 1.4-kg saturated liquid water at 200 oC. Now heat is
transferred to the water until the volume quadruples and the cylinder contains saturated vapor
only. Determine the internal energy change of the water in KJ.
10. A throttling calorimeter is connected to a desuperheated steam line supplying steam to the
auxiliary feed pump on a ship. The line pressure measures 2.5 MPa. The calorimeter pressure is
110 kPa at 150°C. Determine the entropy of the steam line in KJ/Kg-K.
You might also like
- Twenty Kilograms of Water at 40oc Is Confined in A Rigid VesselDocument1 pageTwenty Kilograms of Water at 40oc Is Confined in A Rigid VesselMelindaNo ratings yet
- Tut 1Document2 pagesTut 1GUNJAN MUDGALNo ratings yet
- Tut 1Document2 pagesTut 1me21b105No ratings yet
- Group Quiz ME 63 THYZ - WFWX EntropyDocument2 pagesGroup Quiz ME 63 THYZ - WFWX Entropyluvieduffy_13No ratings yet
- Homework 4 - MEEN 2300 - Spring 2015Document3 pagesHomework 4 - MEEN 2300 - Spring 2015MichaelNo ratings yet
- ThermoDocument5 pagesThermoTerry Clarice DecatoriaNo ratings yet
- PR Closed System 2Document2 pagesPR Closed System 2Mareta DanarNo ratings yet
- Practice Problems On EntropyDocument1 pagePractice Problems On EntropyNetra PujarNo ratings yet
- SAQ 6-ThermodynamicsDocument1 pageSAQ 6-Thermodynamicsjhigs amfufuNo ratings yet
- A Quantity of Steam at 10 Bar and 0Document1 pageA Quantity of Steam at 10 Bar and 0MelindaNo ratings yet
- UES011 Thermofluids (Thermodynamics) : Tutorial Sheet No.4Document2 pagesUES011 Thermofluids (Thermodynamics) : Tutorial Sheet No.4s barmanNo ratings yet
- Pset 1 CombustionDocument13 pagesPset 1 CombustionMicaella Jaime De LeonNo ratings yet
- Sheet #7Document4 pagesSheet #7AHMED BAKRNo ratings yet
- Thermodynamics ProblemsDocument1 pageThermodynamics ProblemsTots HolaresNo ratings yet
- Ejercicios 1a Ley-04-03-2021Document6 pagesEjercicios 1a Ley-04-03-2021Angelica PobladorNo ratings yet
- Tutorial 5 PDFDocument3 pagesTutorial 5 PDFAnonymous hxyDxxcoJNo ratings yet
- ME 63 Prob Set 2Document2 pagesME 63 Prob Set 2RamonVannCleffRaroNo ratings yet
- Lectut MI 106 PDF MI 106 Sol Tut 5 76vs9e5Document4 pagesLectut MI 106 PDF MI 106 Sol Tut 5 76vs9e5Pritam PaulNo ratings yet
- Tutorial 2Document2 pagesTutorial 2Nayli SorfinaNo ratings yet
- Assignment 1Document6 pagesAssignment 1Roshan ShanmughanNo ratings yet
- Thermodynamics Tutorial ProblemsDocument20 pagesThermodynamics Tutorial ProblemsRishabh Sharma100% (1)
- T 2Document1 pageT 2jfl2096No ratings yet
- Availability Analysis: Tutorial QuestionsDocument2 pagesAvailability Analysis: Tutorial QuestionsJackson TeohNo ratings yet
- Assignment Basics ATDocument2 pagesAssignment Basics ATXerox WorldNo ratings yet
- Thermodynamics Review ProblemsDocument3 pagesThermodynamics Review ProblemssayanNo ratings yet
- Course Work Tft-1Document4 pagesCourse Work Tft-1Ahmad HashemNo ratings yet
- Tutorial ThermodynamicsDocument2 pagesTutorial Thermodynamics23f3001728No ratings yet
- Sheet 2Document2 pagesSheet 2Ahmed Rabie Abd Elazeem100% (1)
- Problemas TermoDocument3 pagesProblemas TermocleonardoeNo ratings yet
- Assignment # 4 (Chapter 5) Due 3:00 PM of February 07, 2008 1Document2 pagesAssignment # 4 (Chapter 5) Due 3:00 PM of February 07, 2008 1Adam SchellNo ratings yet
- Tutorial 1Document1 pageTutorial 1Zakaria HassanNo ratings yet
- Taller 2 2023-1Document8 pagesTaller 2 2023-1anderson ortizNo ratings yet
- Practice Problems For Heat ExchangerDocument2 pagesPractice Problems For Heat ExchangerFour AyesNo ratings yet
- Assignment EntropyDocument2 pagesAssignment Entropyme22b009No ratings yet
- ME4202501 Thermodynamics I, Fall Term 2015 Practice 12: 8.314 kJ/kmol-KDocument1 pageME4202501 Thermodynamics I, Fall Term 2015 Practice 12: 8.314 kJ/kmol-K黃羿傑No ratings yet
- T 5Document2 pagesT 5jfl2096No ratings yet
- ThermoDocument4 pagesThermowong zhi chengNo ratings yet
- Assignment 3Document2 pagesAssignment 3Saurabh BhimwalNo ratings yet
- Jawaban No 3Document2 pagesJawaban No 3zidnyNo ratings yet
- XXDocument1 pageXXGetachew TikueNo ratings yet
- XXDocument1 pageXXGetachew TikueNo ratings yet
- Sheet 4 First Law of ThermodynamicsDocument1 pageSheet 4 First Law of Thermodynamicsahmed kaedNo ratings yet
- 2022 Tut 5Document2 pages2022 Tut 5Kethavath Sunil ch21b047No ratings yet
- Moving Boundary Work. 2015Document2 pagesMoving Boundary Work. 2015Brayan Steven Cubillos MorenoNo ratings yet
- Steam ProblemsDocument10 pagesSteam ProblemsCaro Kan LopezNo ratings yet
- Assignment ProblemsDocument2 pagesAssignment ProblemsRam SharmaNo ratings yet
- Assignment 1 Numerical ProblemsDocument3 pagesAssignment 1 Numerical ProblemsNetra PujarNo ratings yet
- Thermodynamics I P 11 PDFDocument1 pageThermodynamics I P 11 PDF黃羿傑No ratings yet
- Numerical 3Document10 pagesNumerical 322210021 TANWADE RUTURAJ RAVINDRANo ratings yet
- Tugas Ke 2 Klas DRM2Document1 pageTugas Ke 2 Klas DRM2loafNo ratings yet
- Department of Biomedical Engineering (Aait) : Work Sheet #3Document4 pagesDepartment of Biomedical Engineering (Aait) : Work Sheet #3gfsfNo ratings yet
- Problems in ExergyDocument2 pagesProblems in ExergyMukul .sNo ratings yet
- Sheet #7Document4 pagesSheet #7Iam A gnoomNo ratings yet
- Tutorial Sheet 6Document2 pagesTutorial Sheet 6Syed YousufuddinNo ratings yet
- Practice Problems On First Law For Closed SystemDocument3 pagesPractice Problems On First Law For Closed SystemNetra PujarNo ratings yet
- Uts TermodinamikaDocument6 pagesUts TermodinamikaFadhillah AnsyariNo ratings yet
- Chapter 6Document18 pagesChapter 6Xeen FortunyNo ratings yet
- Tutorial 4Document2 pagesTutorial 4Diego Cuarenta JaureguiNo ratings yet
- Mechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesFrom EverandMechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesNo ratings yet
- Belt, Rope and Chain Drives Denition: M Arshad Zahangir ChowdhuryDocument12 pagesBelt, Rope and Chain Drives Denition: M Arshad Zahangir ChowdhuryMelindaNo ratings yet
- Question: Two Walls of A Storage Plant Composed of Insulating Material (Document3 pagesQuestion: Two Walls of A Storage Plant Composed of Insulating Material (MelindaNo ratings yet
- Laplace and Inverse Laplace TransformsDocument3 pagesLaplace and Inverse Laplace TransformsMelindaNo ratings yet
- The Heat Transfer Across A 5" Wall of Firebrick Is...Document3 pagesThe Heat Transfer Across A 5" Wall of Firebrick Is...MelindaNo ratings yet
- Linearity: Find The Inverse Laplace Transforms of The Following. F(S) F (T) F(S) F (T)Document3 pagesLinearity: Find The Inverse Laplace Transforms of The Following. F(S) F (T) F(S) F (T)MelindaNo ratings yet
- Question: Need Help On This 2 Questions: An Insulated Steam Pipe LocaDocument4 pagesQuestion: Need Help On This 2 Questions: An Insulated Steam Pipe LocaMelindaNo ratings yet
- Matlab-Integral CalculusDocument1 pageMatlab-Integral CalculusMelindaNo ratings yet
- Activity 1 Emath 222BDocument1 pageActivity 1 Emath 222BMelinda100% (1)
- Plate No. 3 ConvectionDocument1 pagePlate No. 3 ConvectionMelindaNo ratings yet
- Belts and PulleysDocument17 pagesBelts and PulleysMelindaNo ratings yet
- Plate No. 5 Mean Temperature DifferenceDocument1 pagePlate No. 5 Mean Temperature DifferenceMelindaNo ratings yet
- CHAINS and SPROCKETS (Types, Application, Advantages, Disadvantages)Document18 pagesCHAINS and SPROCKETS (Types, Application, Advantages, Disadvantages)MelindaNo ratings yet
- Plate No. 4 RadiationDocument1 pagePlate No. 4 RadiationMelindaNo ratings yet
- Roller Chain SprocketDocument10 pagesRoller Chain SprocketMelindaNo ratings yet
- Plate No. 3 ConvectionDocument1 pagePlate No. 3 ConvectionMelindaNo ratings yet
- Machine Elements 2 (Introduction)Document12 pagesMachine Elements 2 (Introduction)Melinda100% (2)
- Me 323 (P4)Document1 pageMe 323 (P4)MelindaNo ratings yet
- Me 323 (P3)Document3 pagesMe 323 (P3)MelindaNo ratings yet
- Plate No. 4 RadiationDocument1 pagePlate No. 4 RadiationMelindaNo ratings yet
- Spur GearDocument5 pagesSpur GearMelindaNo ratings yet
- Machine DesignDocument15 pagesMachine DesignYaNo ratings yet
- Me 323 (P3)Document3 pagesMe 323 (P3)MelindaNo ratings yet
- Me 323 (P4)Document1 pageMe 323 (P4)MelindaNo ratings yet
- Plate No. 5 Mean Temperature DifferenceDocument1 pagePlate No. 5 Mean Temperature DifferenceMelindaNo ratings yet
- Group 2: Kim Vincent E. Hurboda Ivan Klee Sasan Josh Kendrick Gerasmio Christian M. Baricuatro Elijah C. Suico Lordjie OrtegaDocument32 pagesGroup 2: Kim Vincent E. Hurboda Ivan Klee Sasan Josh Kendrick Gerasmio Christian M. Baricuatro Elijah C. Suico Lordjie Ortegajed likeNo ratings yet
- Coal Distribution Around The WorldDocument9 pagesCoal Distribution Around The Worldjed likeNo ratings yet
- Elements of Mechanism Work Book and Lab ManualDocument105 pagesElements of Mechanism Work Book and Lab ManualMelinda100% (1)
- Experiment No. 5 - Measurement of SpeedDocument7 pagesExperiment No. 5 - Measurement of SpeedMelindaNo ratings yet
- Me 325 QuizDocument1 pageMe 325 QuizMelindaNo ratings yet
- Me Formulas and Review ManualDocument162 pagesMe Formulas and Review ManualLynel Arianne TaborNo ratings yet