Professional Documents
Culture Documents
ALTECH PP-H A 2030/750 GF30 CP: Technical Data Sheet
Uploaded by
Gufran AhmadOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ALTECH PP-H A 2030/750 GF30 CP: Technical Data Sheet
Uploaded by
Gufran AhmadCopyright:
Available Formats
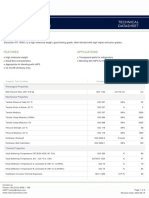
Technical Data Sheet
ALTECH PP-H A 2030/750 GF30 CP
(Last update: 06.01.2021)
Base Polymer Polypropylene Homopolymer
Filler/Additive System 30 % glass fibres
Special Features chemically coupled, high heat stabilised, high stiffness, high toughness
Market Segment Automotive
Typical Applications various
Pre-Drying Conditions in a dry air (dessiccant) dryer 80-120 °C
for 2-3 h
in an air circulating dryer 80-120 °C
for 2-4 h
Processing Conditions injection moulding melt temperature 200-270 °C
injection moulding mould temperature 20-90 °C
Storage dry, protected from light
not above 30°C
Properties Value Dimension Test Norm
Mechanical Properties
Flexural Modulus 5600 MPa ISO 178
Flexural Strength 125 MPa ISO 178
Tensile Modulus 7100 MPa ISO 527
Tensile Strength at Break 90 MPa ISO 527
Tensile Elongation at Break 3.3 % ISO 527
Impact Strength (Charpy, 23°C) 55 kJ/m² ISO 179/1eU
Impact Strength (Charpy, -40°C) 45 kJ/m² ISO 179/1eU
Notched Impact Strength (Charpy, 23°C) 10 kJ/m² ISO 179/1eA
Notched Impact Strength (Charpy, -40°C) 8 kJ/m² ISO 179/1eA
Thermal Properties
Vicat B50 134 °C ISO 306
HDT / A (1,8 MPa) 150 °C ISO 75-1/-2
DSC (Melt Point) 168 °C ISO 11357
Rheological Properties
Melt Index (MVR) 5 cm³/10min ISO 1133
MVR temperature 230 °C -
MVR load 2.16 kg -
Shrinkage (lengthwise, 24h) 0.2 - 0.4 % ISO 294-4
Shrinkage (lateral, 24h) 0.4 - 0.6 % ISO 294-4
Physical Properties
Density 1160 kg/m³ ISO 1183
ALBIS Distribution GmbH & Co. KG Phone: + 49 (0) 40 78105-0 www.albis.com
TDS16272 Print Date: 2021-04-12 09:39:58 Page 1 of 2
Technical Data Sheet
ALTECH PP-H A 2030/750 GF30 CP
(Last update: 06.01.2021)
These are guide values and not a specification. The test values mentioned are representative values only and not binding minimum or
maximum figures. These test values have been determined on standardised test specimens and can be affected by pigmentation, mould
design and processing conditions.
Any information given on the chemical and physical characteristics of our products, including, without limitation, technical advice on
applications, whether verbally, in writing or by testing the product, is given to the best of our knowledge and in good faith and does not
exempt the buyer from carrying out their own investigations and tests in order to ascertain the product's specific suitability for the purpose
intended.
The buyer is solely responsible for confirming the suitability of the product for a particular application, its utilization and processing and
must observe any applicable laws and government regulations. NO EXPRESS OR IMPLIED RECOMMENDATION OR WARRANTY IS
GIVEN WITH REGARD TO THE SUITABILITY OF THE PRODUCT FOR A PARTICULAR APPLICATION, SUCH AS, BUT NOT
LIMITED TO, SAFETY-CRITICAL COMPONENTS OR SYSTEMS.
Healthcare uses: the supply of any product by ALBIS for any medical, pharmaceutical or diagnostic application is conditional to an
assessment by ALBIS in terms of compliance with ALBIS’ internal risk management policy – even for products which are in general
designated for use in Healthcare applications.
Important: irrespective of product type or designation, ALBIS does not recommend or support the use of any products it supplies which fall
into the following medical, pharmaceutical or diagnostic application categories:
risk class III applications according to EU directive 93/42/EEC
any bodily implant application for greater than 30 days
any critical component in any medical device that supports or sustains human life.
At all times, our standard terms and conditions of sale apply.
ALBIS Distribution GmbH & Co. KG Phone: + 49 (0) 40 78105-0 www.albis.com
TDS16272 Print Date: 2021-04-12 09:39:58 Page 2 of 2
You might also like
- VW Golf 7 2015 Suspension Steering EngDocument375 pagesVW Golf 7 2015 Suspension Steering Engcloantaf100% (1)
- Injection Molding Scrap ReductionDocument57 pagesInjection Molding Scrap ReductionkggganiNo ratings yet
- Performance Analysis of Cooling TowerDocument7 pagesPerformance Analysis of Cooling TowerIbrahim Al-MutazNo ratings yet
- Underwater Cutting and Welding Equipment - 89250054 - AbDocument38 pagesUnderwater Cutting and Welding Equipment - 89250054 - AbAhmed Adel100% (1)
- GE Design GuideDocument53 pagesGE Design GuideGuido Kats100% (1)
- Autodesk Inventor Practice Part DrawingsDocument25 pagesAutodesk Inventor Practice Part DrawingsCiprian Fratila100% (1)
- L1 Finding Nemo Teacher Notes American EnglishDocument9 pagesL1 Finding Nemo Teacher Notes American Englishcris_simescuNo ratings yet
- IV Solution Cheat Sheet: Type Description Osmolality Use MiscellaneousDocument1 pageIV Solution Cheat Sheet: Type Description Osmolality Use MiscellaneousKristine Castillo100% (2)
- Iso 4309 2017Document10 pagesIso 4309 2017C. de JongNo ratings yet
- Autocad ShortcutsDocument13 pagesAutocad ShortcutsKriscel CaraanNo ratings yet
- DL Throughput Improvement with Aperiodic CQI ReportingDocument10 pagesDL Throughput Improvement with Aperiodic CQI ReportingAqeel HasanNo ratings yet
- Technical Data Sheet for ALTECH PP-H A 4920/200 TV20 PolypropyleneDocument2 pagesTechnical Data Sheet for ALTECH PP-H A 4920/200 TV20 PolypropyleneDjaafar DerguiniNo ratings yet
- ALTECH PE-HD A 2010/550 GF10: Technical Data SheetDocument2 pagesALTECH PE-HD A 2010/550 GF10: Technical Data SheetPhung LucNo ratings yet
- ALTECH PE-HD A 2010/506 GF10: Technical Data SheetDocument2 pagesALTECH PE-HD A 2010/506 GF10: Technical Data SheetPhung LucNo ratings yet
- Material DataDocument3 pagesMaterial DataR.Ranjan PradhanNo ratings yet
- ABS Technical Data SheetDocument1 pageABS Technical Data SheetarmandoNo ratings yet
- ULTRAMID_sup_®️__sup_+B3WG6+BGVW+BLACK+00564 (1)Document2 pagesULTRAMID_sup_®️__sup_+B3WG6+BGVW+BLACK+00564 (1)Luis Enrique Ramos PérezNo ratings yet
- Data SheetDocument2 pagesData SheetDridi BadreddineNo ratings yet
- Technical Data Sheet for ALTECH ABS C 2017/500 GF17 PlasticDocument1 pageTechnical Data Sheet for ALTECH ABS C 2017/500 GF17 PlasticarmandoNo ratings yet
- ABS Terlux 2802 TR - Fiche Technique enDocument2 pagesABS Terlux 2802 TR - Fiche Technique endamien_roule5728No ratings yet
- Technical Data Sheet - TheRMOFIL PP F820R00 Natural-Sumika Polymer Compounds Ltd. (2017)Document2 pagesTechnical Data Sheet - TheRMOFIL PP F820R00 Natural-Sumika Polymer Compounds Ltd. (2017)Priyalakshmi NarasimhanNo ratings yet
- Ultradur® B 6550 LN en SI - Product DatasheetDocument2 pagesUltradur® B 6550 LN en SI - Product Datasheetnoto.sugiartoNo ratings yet
- PDS HEX4460p PE80 1Document3 pagesPDS HEX4460p PE80 1art72 talNo ratings yet
- Latigloss 66 H2 G 50 F2 PDFDocument4 pagesLatigloss 66 H2 G 50 F2 PDFBadis ChemaliNo ratings yet
- Ultradur: Product InformationDocument3 pagesUltradur: Product InformationirisNo ratings yet
- Tds Tasnee 100 Black Revised in 2022Document2 pagesTds Tasnee 100 Black Revised in 2022Amir NawazNo ratings yet
- Pa6 GF15 - Basf Ultramid B3eg3Document2 pagesPa6 GF15 - Basf Ultramid B3eg3armandoNo ratings yet
- Caring Formula High Flow Glass Filled PA6Document3 pagesCaring Formula High Flow Glass Filled PA6ratz23695No ratings yet
- Ultramid® B3S en SIDocument2 pagesUltramid® B3S en SImuamerNo ratings yet
- Technical Data SheetDocument3 pagesTechnical Data SheetAdrián SánchezNo ratings yet
- Borouge HD168MODocument2 pagesBorouge HD168MOhamza hameedNo ratings yet
- Domamid 6hvi4h2 BK - en TDSDocument1 pageDomamid 6hvi4h2 BK - en TDSMadhav RajpurohitNo ratings yet
- Updated TDS-TASNEE 100 OrangeDocument2 pagesUpdated TDS-TASNEE 100 Orangelamia.elgammalNo ratings yet
- ULTRASON Sup ® Sup +E1010+NATURALDocument2 pagesULTRASON Sup ® Sup +E1010+NATURALnikos.a.kyriakouNo ratings yet
- PP GF30 - Borealis Gb311uDocument3 pagesPP GF30 - Borealis Gb311uarmandoNo ratings yet
- Technical Data SheetDocument3 pagesTechnical Data SheetEmanuel TescheNo ratings yet
- Tasnee LD 1925as: Low Density PolyethyleneDocument2 pagesTasnee LD 1925as: Low Density PolyethyleneMootaz Nagy El SabaaNo ratings yet
- VG621 PPGF30 BorealisDocument3 pagesVG621 PPGF30 BorealisLeandro LacerdaNo ratings yet
- Ultrason® E 2010 G6 UN en SI - Product DatasheetDocument2 pagesUltrason® E 2010 G6 UN en SI - Product Datasheetshahin_723No ratings yet
- Styrolution PS 454N: High Impact Polystyrene (HIPS)Document3 pagesStyrolution PS 454N: High Impact Polystyrene (HIPS)Inês MorgadoNo ratings yet
- C C14 LD2420F PlusDocument3 pagesC C14 LD2420F PlusJaime222No ratings yet
- Technyl Safe C 116FC NCDocument3 pagesTechnyl Safe C 116FC NCkls.thorodinsonNo ratings yet
- Product Data Sheet: Sasol Polymers PP: HNR100 MFR: 12g/10minDocument2 pagesProduct Data Sheet: Sasol Polymers PP: HNR100 MFR: 12g/10minAlbert FortunatoNo ratings yet
- PP Omopolimero - Borealis Hd120mo TDSDocument2 pagesPP Omopolimero - Borealis Hd120mo TDSarmandoNo ratings yet
- Styrolution PS 165N/L: General Purpose Polystyrene (GPPS)Document3 pagesStyrolution PS 165N/L: General Purpose Polystyrene (GPPS)Inês MorgadoNo ratings yet
- Ultradur B2520Document2 pagesUltradur B2520Phung LucNo ratings yet
- Tds-Tasnee LD 0725nDocument2 pagesTds-Tasnee LD 0725nأبو أميرNo ratings yet
- RB206MODocument2 pagesRB206MObobNo ratings yet
- Econamid FL 6B30Document2 pagesEconamid FL 6B30Jagadeesh WaranNo ratings yet
- Kepital F20 - 03Document2 pagesKepital F20 - 03Kumaar RanjanNo ratings yet
- Absolac 300 Ineos AbsDocument2 pagesAbsolac 300 Ineos Absadrian4santanaNo ratings yet
- Styrolution PS 485N: High Impact Polystyrene (HIPS)Document2 pagesStyrolution PS 485N: High Impact Polystyrene (HIPS)Inês MorgadoNo ratings yet
- CovestroDocument2 pagesCovestroRonaldo CamargoNo ratings yet
- Styro Lution Tds 400900240714Document3 pagesStyro Lution Tds 400900240714vrjrNo ratings yet
- High Density Polyethylene Product Data SheetDocument2 pagesHigh Density Polyethylene Product Data Sheetchirag.sanchetiNo ratings yet
- Ultramid: Product Information ®Document2 pagesUltramid: Product Information ®muthuNo ratings yet
- 0210 Ibd - MSDS Reg - Europe en V4 ZPDS Eur 48808 10038429Document3 pages0210 Ibd - MSDS Reg - Europe en V4 ZPDS Eur 48808 10038429Mehdi SaouriNo ratings yet
- Tds Tasnee HD b1258Document2 pagesTds Tasnee HD b1258أبو أميرNo ratings yet
- TITLE Fast Molding Polyamide for Thin Wall PartsDocument3 pagesTITLE Fast Molding Polyamide for Thin Wall PartsSATHISH KUMAR SNo ratings yet
- CR C14 LD2420KDocument3 pagesCR C14 LD2420KJaime222No ratings yet
- CirRenew C14 LD2420DDocument3 pagesCirRenew C14 LD2420DJaime222No ratings yet
- Lyondell Basell Moplen EP548PDocument4 pagesLyondell Basell Moplen EP548PIndrajit SahaNo ratings yet
- Styrolution PS 495N: High Impact Polystyrene (HIPS)Document3 pagesStyrolution PS 495N: High Impact Polystyrene (HIPS)Inês MorgadoNo ratings yet
- Tds Tasnee HD Im2050 Revised in 2022Document2 pagesTds Tasnee HD Im2050 Revised in 2022Amir NawazNo ratings yet
- Hifax: Technical Data SheetDocument3 pagesHifax: Technical Data SheetaLe08ajNo ratings yet
- Econamid FL 6M20 - Pa6 M2oDocument1 pageEconamid FL 6M20 - Pa6 M2omuthuNo ratings yet
- Styrolution TDS400700190196Document3 pagesStyrolution TDS400700190196asegundoferreiraNo ratings yet
- VG602Document2 pagesVG602LucasNo ratings yet
- Technical Data Sheet ADFLEX F 500Document2 pagesTechnical Data Sheet ADFLEX F 500leftpowtaNo ratings yet
- PP h4120 TdsDocument2 pagesPP h4120 TdsengrmfawadazharNo ratings yet
- Design and Construction of Two Plate Mould for Cellotape FrameDocument60 pagesDesign and Construction of Two Plate Mould for Cellotape FrameGufran AhmadNo ratings yet
- MoldingDocument33 pagesMoldingAnurag Srivastava100% (1)
- 14 Arbor PressDocument16 pages14 Arbor PressGufran AhmadNo ratings yet
- Solid WorksDocument33 pagesSolid WorksAbdallah MohammedNo ratings yet
- Autocad LT Shortcuts & Hotkey GuideDocument11 pagesAutocad LT Shortcuts & Hotkey GuideAryanNo ratings yet
- SolidWorks Education Detailed Drawing ExercisesDocument51 pagesSolidWorks Education Detailed Drawing ExercisesGufran AhmadNo ratings yet
- Mechanical Properties of Polypropylene Composites A Review: Journal of Thermoplastic Composite Materials April 2013Document31 pagesMechanical Properties of Polypropylene Composites A Review: Journal of Thermoplastic Composite Materials April 2013Gufran AhmadNo ratings yet
- KL Jack Fasteners-Technical Data and ChartsDocument32 pagesKL Jack Fasteners-Technical Data and ChartsGufran AhmadNo ratings yet
- Research ArticleDocument9 pagesResearch ArticleGufran AhmadNo ratings yet
- Research ArticleDocument9 pagesResearch ArticleGufran AhmadNo ratings yet
- Template Contoh HSE PlanDocument6 pagesTemplate Contoh HSE PlandulNo ratings yet
- ALTECH PP-H A 2030/750 GF30 CP: Technical Data SheetDocument2 pagesALTECH PP-H A 2030/750 GF30 CP: Technical Data SheetGufran AhmadNo ratings yet
- Physical and Mechanical Properties of Polypropylene Reinforced With Fe ParticlesDocument9 pagesPhysical and Mechanical Properties of Polypropylene Reinforced With Fe ParticlesGufran AhmadNo ratings yet
- Template Contoh HSE PlanDocument6 pagesTemplate Contoh HSE PlandulNo ratings yet
- Template Contoh HSE PlanDocument6 pagesTemplate Contoh HSE PlandulNo ratings yet
- Mechanical Properties of Polypropylene Composites A Review: Journal of Thermoplastic Composite Materials April 2013Document31 pagesMechanical Properties of Polypropylene Composites A Review: Journal of Thermoplastic Composite Materials April 2013Gufran AhmadNo ratings yet
- Mechanical Properties of Polypropylene Composites A Review: Journal of Thermoplastic Composite Materials April 2013Document31 pagesMechanical Properties of Polypropylene Composites A Review: Journal of Thermoplastic Composite Materials April 2013Gufran AhmadNo ratings yet
- Presidential Decree No. 1616 establishes Intramuros AdministrationDocument22 pagesPresidential Decree No. 1616 establishes Intramuros AdministrationRemiel Joseph Garniel BataoNo ratings yet
- Different Project Topics of BSNLDocument3 pagesDifferent Project Topics of BSNLAbhijit Tripathy0% (1)
- White Chicken KormaDocument5 pagesWhite Chicken Kormamkm2rajaNo ratings yet
- 60 Minutes-60 Questions: Mathematics TestDocument15 pages60 Minutes-60 Questions: Mathematics TestJihyun YeonNo ratings yet
- E32-433T30D User Manual: Sx1278 433Mhz 1W Dip Wireless ModuleDocument22 pagesE32-433T30D User Manual: Sx1278 433Mhz 1W Dip Wireless ModuleSergey SevruginNo ratings yet
- Towards Sustainable Water Management in SoharDocument48 pagesTowards Sustainable Water Management in SoharMiss Preyashi kumarNo ratings yet
- Accounting For AC Winding Losses in The Electric Machine Design ProcessDocument4 pagesAccounting For AC Winding Losses in The Electric Machine Design Processjianfeng wangNo ratings yet
- 22nd Annual Report 2021-22Document155 pages22nd Annual Report 2021-22Karthic Selvam KandavelNo ratings yet
- Intel Processors PDFDocument33 pagesIntel Processors PDFbiplab royNo ratings yet
- Model AnswerDocument27 pagesModel AnswerdishaNo ratings yet
- Phylum Chordata TransesDocument2 pagesPhylum Chordata TransesMaribel Ramos InterinoNo ratings yet
- Unit 1.Pptx Autosaved 5bf659481837fDocument39 pagesUnit 1.Pptx Autosaved 5bf659481837fBernadith Manaday BabaloNo ratings yet
- PWR Bi2Document11 pagesPWR Bi2GOMTINo ratings yet
- Strength of CSG and TBGDocument10 pagesStrength of CSG and TBGTiffany DacinoNo ratings yet
- Mbbs BooksDocument7 pagesMbbs Booksbakhem7hbk2002190% (1)
- Mushroom Umami Taste EvaluationDocument10 pagesMushroom Umami Taste EvaluationMaryam HanifNo ratings yet
- Home Automation Chapter 1Document7 pagesHome Automation Chapter 1Nishant Sawant100% (1)
- The Human BodyDocument17 pagesThe Human BodyRuthie MendozaNo ratings yet
- La Consolacion College - Caloocan: Module in General Physics 1 Module 5: Kinematic QuantitiesDocument6 pagesLa Consolacion College - Caloocan: Module in General Physics 1 Module 5: Kinematic QuantitiesJhon Christian ManzoNo ratings yet
- All Questions SLDocument50 pagesAll Questions SLRoberto Javier Vázquez MenchacaNo ratings yet
- Assignment 1 (Total Marks: 70) EEE 323: Power System IIDocument2 pagesAssignment 1 (Total Marks: 70) EEE 323: Power System IIShahriar SauravNo ratings yet
- 3 Uscg BWM VRPDocument30 pages3 Uscg BWM VRPdivinusdivinusNo ratings yet
- Architecture in The AnthropoceneDocument265 pagesArchitecture in The Anthropoceneziyad fauziNo ratings yet