0% found this document useful (0 votes)
168 views1 pageUniversal Indicator Guide
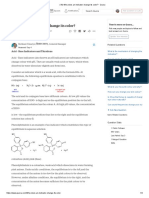
A universal indicator is a pH indicator solution made up of multiple compounds that changes color smoothly over a wide range of pH values, indicating whether a solution is acidic, alkaline, or neutral. It is usually composed of water, 1-propanol, phenolphthalein, sodium hydroxide, methyl red, bromothymol blue, sodium bisulfite, and thymol blue. Different colors indicate different pH ranges from acidic to alkaline, with green indicating a neutral pH of 7. Universal indicators allow for easy determination of solution acidity across a broad range of pH levels with a single test.
Uploaded by
Noha ShaabanCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
168 views1 pageUniversal Indicator Guide
A universal indicator is a pH indicator solution made up of multiple compounds that changes color smoothly over a wide range of pH values, indicating whether a solution is acidic, alkaline, or neutral. It is usually composed of water, 1-propanol, phenolphthalein, sodium hydroxide, methyl red, bromothymol blue, sodium bisulfite, and thymol blue. Different colors indicate different pH ranges from acidic to alkaline, with green indicating a neutral pH of 7. Universal indicators allow for easy determination of solution acidity across a broad range of pH levels with a single test.
Uploaded by
Noha ShaabanCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd