Professional Documents
Culture Documents
Practical 8: Volumetric Analysis
Practical 8: Volumetric Analysis
Uploaded by
Joshua-Jonai JulyeOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Practical 8: Volumetric Analysis
Practical 8: Volumetric Analysis
Uploaded by
Joshua-Jonai JulyeCopyright:
Available Formats
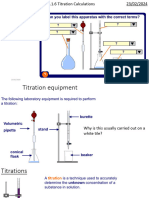
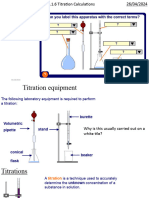
Practical 8: VOLUMETRIC ANALYSIS
Aim: To determine the concentration of sodium hydroxide by titration
Apparatus/Material: Pipette, burette, clamp stand, 0.1M Sulphuric acid, Sodium hydroxide,
white tile, funnel, beakers, distilled water, wash bottles, white paper, methyl orange
Method:
1. H SO was placed into a burette with the tip/jet filled and the initial volume recorded.
2 4
2. A pipette was filled up to its graduated mark with the solution NaOH and was then
transferred to a 250cm3 conical flask.
3. The acid solution was titrated against the alkali solution until one drop had caused a
change in colour
4. The volume of H SO that was left in the burette was recorded and it was used to
2 4
determine the volume of H2SO4 that was added.
5. The titration was repeated until two readings that were within at least 0.1cm3 of each
other were found.
6. The results from the titration were recorded in table form.
Observations:
Results:
Rough 1 2
Final Burette Reading/cm 3
14.00 11.49 22.89
Initial Burette Reading/cm 3
0.10 0.10 11.49
Volume of H SO used/cm
2 4
3
13.90 11.39 11.40
Calculations:
1. Calculate the average volume of H SO ? 2 4
Average vol = (11.39cm3 + 11.40cm3)/2 = 11.40
2. What is the concentration of H SO provided? 2 4
0.01moldm-3
3. What is the volume of the pipette used in the experiment?
25cm3
4. Write a balanced equation for the reaction between H SO and NaOH. 2 4
H2SO4(aq) + 2NaOH(aq) Na2SO4(aq) + 2H2O(l)
5. Determine the mole ratio from the equation.
Mole Ratio: 1 mol of sulphuric acid reacts with 2 mol sodium hydroxide to form 1 mol
sodium sulphate and 2 mol water
6. Determine the number of moles of H SO used. 2 4
Moles of Sulfuric acid = Molar concentration * average volume(dm-3)
= 0.1moldm-3 * 11.40/1000
.00114mol
7. Determine the number of moles in pipetted volume of NaOH.
Since 1 mol H2SO4 reacts with 2 mol NaOH then, 0.00114mol H2SO4 will react with 2
*0.00114mol of NaOH = 0.00228
8. Determine the molar concentration of the NaOH solution.
Concentration of NaOH = molar concentration = moles/volume(dm-3)
= 0.00228mol / (25/1000) dm3
= 0.0912
Conclusion:
You might also like
- Unit 4 - Activity 7 Titration GizmoDocument5 pagesUnit 4 - Activity 7 Titration GizmoSijie Li100% (4)
- Nitrogen Determination by Kjeldahl MethodDocument7 pagesNitrogen Determination by Kjeldahl MethodLinh VũNo ratings yet
- Chemical Compatibility Chart PDFDocument1 pageChemical Compatibility Chart PDFAdjira Sayad100% (1)
- Chemistry Practical For Class 12thDocument19 pagesChemistry Practical For Class 12thVivek77% (121)
- Experiment 7 - Gravimetric Determination of Aluminum As OxinateDocument2 pagesExperiment 7 - Gravimetric Determination of Aluminum As OxinateSavita ChemistryNo ratings yet
- Amount of Substance 4: © WWW - CHEMSHEETS.co - Uk 08-April-2020 Chemsheets AS 1247 1Document4 pagesAmount of Substance 4: © WWW - CHEMSHEETS.co - Uk 08-April-2020 Chemsheets AS 1247 1Ahmad RazaNo ratings yet
- Volumetric Analysis LabDocument3 pagesVolumetric Analysis LabHobi and Jimin’s waRM Jinger Tae with Suga KookiesNo ratings yet
- Experiment 1 The Potentiometric Titration of Hydrogen PeroxideDocument10 pagesExperiment 1 The Potentiometric Titration of Hydrogen PeroxideAfiqah SamanNo ratings yet
- Aswani Forrest CHEM LAB 10Document2 pagesAswani Forrest CHEM LAB 10aswaniNo ratings yet
- 2 Na OHDocument2 pages2 Na OHVenus MitchellNo ratings yet
- Experiment 1 Titration ChemistryDocument4 pagesExperiment 1 Titration ChemistryfitsNo ratings yet
- Chemistry Lab 13Document7 pagesChemistry Lab 13Nathaniel MorrisonNo ratings yet
- Lab 6Document4 pagesLab 6KayenNo ratings yet
- Volumetric Analysis LabDocument2 pagesVolumetric Analysis LabOkera JamesNo ratings yet
- Chemistry Unit One LabsDocument14 pagesChemistry Unit One Labscarlissia wilkinsNo ratings yet
- Acid & Base Part 2Document14 pagesAcid & Base Part 2Chaeyeon KIMNo ratings yet
- PracDocument31 pagesPracapi-3737745100% (1)
- SKL Lab Report 2Document10 pagesSKL Lab Report 2Nisha Lauren VishvanathNo ratings yet
- UntitledDocument2 pagesUntitledBrado BradoNo ratings yet
- Experiment 7Document5 pagesExperiment 7Glen OrrettNo ratings yet
- Exp 044Document5 pagesExp 044daudiali2002No ratings yet
- Acid Base TitrationDocument7 pagesAcid Base TitrationAinul Diyana100% (1)
- 424534X 1SCI20 AT2 SF Titration SinghDocument2 pages424534X 1SCI20 AT2 SF Titration Singhsing0923No ratings yet
- Running Head: CHEMISTRY 1Document5 pagesRunning Head: CHEMISTRY 1Ludwig GeoffreyNo ratings yet
- Titration CalculationsDocument52 pagesTitration Calculationswilliam.ongeri.tutoringNo ratings yet
- Titration of HCL With NaohDocument9 pagesTitration of HCL With Naohapi-295783672No ratings yet
- Lab #4 - FinalDocument8 pagesLab #4 - FinalEmmaNo ratings yet
- 1 1 3 Acids SG 2014Document10 pages1 1 3 Acids SG 2014steven12345No ratings yet
- Lab Report On Effect of Concentration of Acid Used On Volume of Base Required To Neutralise SolutionDocument9 pagesLab Report On Effect of Concentration of Acid Used On Volume of Base Required To Neutralise SolutionSaransh JainNo ratings yet
- Practical XI Class 11 ChemistryDocument18 pagesPractical XI Class 11 ChemistryTechno GuruNo ratings yet
- Titration CalculationsDocument52 pagesTitration Calculationswilliam.ongeri.tutoringNo ratings yet
- Unit 11 Titration LabDocument2 pagesUnit 11 Titration LabKaran Sumeet Shetty100% (1)
- Chemistry Practical 2022 - XIIDocument21 pagesChemistry Practical 2022 - XIIAayanurNo ratings yet
- Laboratory Experiment No.9 - Neutralization TitrationDocument4 pagesLaboratory Experiment No.9 - Neutralization TitrationShayne Angelique CongsonNo ratings yet
- Lab Report 3 Chemistry 109 PDFDocument2 pagesLab Report 3 Chemistry 109 PDFbayzidm22201415935No ratings yet
- Experiment 5 ChemistryDocument3 pagesExperiment 5 ChemistryJack OngNo ratings yet
- 1112 Grade 12 Chemistry Revision Sheet Final Term 2Document32 pages1112 Grade 12 Chemistry Revision Sheet Final Term 2aalharthy_1No ratings yet
- Chemistry Laboratory Report PH Scale andDocument8 pagesChemistry Laboratory Report PH Scale andM.NASIRNo ratings yet
- CHM 153 - Exp 4Document6 pagesCHM 153 - Exp 4hafiqahNo ratings yet
- FinalExam Sample Problems - 081711Document4 pagesFinalExam Sample Problems - 081711aNo ratings yet
- Analysis of Hydrogen PeroxideDocument6 pagesAnalysis of Hydrogen PeroxideAhmad AlhamwiNo ratings yet
- Experiment 1 2015Document4 pagesExperiment 1 2015UngHHNo ratings yet
- Titration (Practical Test) Group 6Document4 pagesTitration (Practical Test) Group 6Syahminazuhan KhamisNo ratings yet
- Lab Report - Titration of Hydrochloric Acid With Sodium Hydroxide 2Document13 pagesLab Report - Titration of Hydrochloric Acid With Sodium Hydroxide 2api-644259218No ratings yet
- Acid/Alkali TitrationDocument2 pagesAcid/Alkali TitrationumisherruNo ratings yet
- Laporan ResmiacidialkalisudahperiksaDocument15 pagesLaporan ResmiacidialkalisudahperiksaKevan Alvian HartonoNo ratings yet
- Tee Biochem Writeup 1Document7 pagesTee Biochem Writeup 1Tinotenda ChiwengaNo ratings yet
- University Distrital Francisco Jose of Caldas Faculty of Sciences and Education Master in Chemistry 2013Document4 pagesUniversity Distrital Francisco Jose of Caldas Faculty of Sciences and Education Master in Chemistry 2013Diana RoaNo ratings yet
- Preparation For Chemistry TA3 (Practical)Document9 pagesPreparation For Chemistry TA3 (Practical)leekicia123No ratings yet
- Biochemistry ReportDocument7 pagesBiochemistry Reportnlsyamimijeswi100% (1)
- Chemical AnalysisDocument7 pagesChemical AnalysisSaher BashirNo ratings yet
- Soil Lab Report Experiment No 1-2..Document13 pagesSoil Lab Report Experiment No 1-2..masterofdeath699No ratings yet
- Experiment 6 & 7Document10 pagesExperiment 6 & 7gajenraoNo ratings yet
- Experiment - H2SO4 Titration With NaOHDocument5 pagesExperiment - H2SO4 Titration With NaOHfreeharshaNo ratings yet
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)
- Critical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsFrom EverandCritical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsNo ratings yet
- Tables of the Velocity of Sound in Sea Water: Mathematical Tables SeriesFrom EverandTables of the Velocity of Sound in Sea Water: Mathematical Tables SeriesRating: 5 out of 5 stars5/5 (1)
- International Thermodynamic Tables of the Fluid State, Argon, 1971: Division of Physical Chemistry, Commission on Thermodynamics and Thermochemistry, Thermodynamic Tables ProjectFrom EverandInternational Thermodynamic Tables of the Fluid State, Argon, 1971: Division of Physical Chemistry, Commission on Thermodynamics and Thermochemistry, Thermodynamic Tables ProjectNo ratings yet
- O Level Biology Practice For Structured Questions RespirationFrom EverandO Level Biology Practice For Structured Questions RespirationRating: 4 out of 5 stars4/5 (2)
- Powercrete® R-95: Product Data SheetDocument2 pagesPowercrete® R-95: Product Data SheetFernando Ytozu TairaNo ratings yet
- Part 2 Microscopic World (I) LQ AnswersDocument17 pagesPart 2 Microscopic World (I) LQ AnswersWing LamNo ratings yet
- General Chemistry 1 Quarter 1 - Week 4 (Limiting and Excess Reagents)Document3 pagesGeneral Chemistry 1 Quarter 1 - Week 4 (Limiting and Excess Reagents)Sachzelle MikaylaNo ratings yet
- A Technique For Estimating The Shaft Resistance of Test Piles in Unsaturated SoilsDocument7 pagesA Technique For Estimating The Shaft Resistance of Test Piles in Unsaturated SoilsjohndimNo ratings yet
- Bevel Gearboxes: Product Selection GuideDocument12 pagesBevel Gearboxes: Product Selection GuideGururaja TantryNo ratings yet
- Alkane and AlkeneDocument1 pageAlkane and AlkenegopipooganNo ratings yet
- Bp2023 - Volume IVDocument964 pagesBp2023 - Volume IVGIGI ROCIO HARO MARIÑOS100% (1)
- Physical Chemistry - R. L. MadanDocument1 pagePhysical Chemistry - R. L. MadanOscar Santos EstofaneroNo ratings yet
- International Journal of Research PublicationsDocument7 pagesInternational Journal of Research PublicationsMaricel D. RanjoNo ratings yet
- ARSONDocument4 pagesARSONangelica naquitaNo ratings yet
- JPBA2010 Efficientandeconomical HPLCfulltextDocument10 pagesJPBA2010 Efficientandeconomical HPLCfulltextHonorary UceNo ratings yet
- BIO-RAD Biotechnology 2014 FEMGDocument484 pagesBIO-RAD Biotechnology 2014 FEMGFELIPE EDMUNDO MARTINEZ GONZALEZNo ratings yet
- To Investigate The Effects of The Organic Solvents (10% Ethanol, 30% Ethanol, 50% Ethanol, Chloroform and Paraffin On The Permeability of Cell Membrane of Beetroot.Document3 pagesTo Investigate The Effects of The Organic Solvents (10% Ethanol, 30% Ethanol, 50% Ethanol, Chloroform and Paraffin On The Permeability of Cell Membrane of Beetroot.Samsamsam Hong60% (5)
- DNA-based Self-Assembly of Fluorescent NanodiamondsDocument4 pagesDNA-based Self-Assembly of Fluorescent NanodiamondsPetr CiglerNo ratings yet
- NCERT Solutions For Class 6 April 3 Science Chapter 5 Separation of SubstancesDocument3 pagesNCERT Solutions For Class 6 April 3 Science Chapter 5 Separation of SubstancesPravin PatilNo ratings yet
- The Alchemists Handbook A Practical Manual-19Document1 pageThe Alchemists Handbook A Practical Manual-19Joseph L. WalkerNo ratings yet
- Acetaminophen Nhóm 4Document28 pagesAcetaminophen Nhóm 4Lộc Nguyễn LêNo ratings yet
- Kem Microconcrete - NDocument2 pagesKem Microconcrete - NIrfan BiradarNo ratings yet
- Pigment Printing of TextilesDocument45 pagesPigment Printing of Textilesyadi haryadiNo ratings yet
- Experiment No. 1 - Acids Bases and Buffers 1Document2 pagesExperiment No. 1 - Acids Bases and Buffers 1Raven GoseNo ratings yet
- Chevron Phillips Marlex D139Document1 pageChevron Phillips Marlex D139AthonioMourinhoNo ratings yet
- Development and Validation of RP-HPLC MeDocument5 pagesDevelopment and Validation of RP-HPLC Memelimeli106No ratings yet
- Sec 5 Part 3 CementDocument5 pagesSec 5 Part 3 CementGonz FerolinoNo ratings yet
- Unit 2 Assignment 6: Moles Activity & PracticeDocument2 pagesUnit 2 Assignment 6: Moles Activity & PracticenicolNo ratings yet
- 1 s2.0 S1773224723005725 MainDocument28 pages1 s2.0 S1773224723005725 MainФилип ЈовановскиNo ratings yet
- ARL 3460 - Application - Notes - IronDocument6 pagesARL 3460 - Application - Notes - IronM FieldsNo ratings yet
- Student Exploration: Solubility and TemperatureDocument4 pagesStudent Exploration: Solubility and TemperatureShashaank SharmaNo ratings yet