Professional Documents
Culture Documents
Unit 1 Solid State Just Introduction
Unit 1 Solid State Just Introduction
Uploaded by
Jothis Jose0 ratings0% found this document useful (0 votes)
5 views1 pageThe document discusses the classification of solids into crystalline and amorphous solids. Crystalline solids have a regular, repeating arrangement of particles with long-range order that gives them anisotropic properties and sharp melting points. Examples include NaCl and diamond. In contrast, amorphous solids have an irregular, random arrangement of particles with only short-range order, making them isotropic with a range of melting points. Plastic and glass are amorphous solids.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses the classification of solids into crystalline and amorphous solids. Crystalline solids have a regular, repeating arrangement of particles with long-range order that gives them anisotropic properties and sharp melting points. Examples include NaCl and diamond. In contrast, amorphous solids have an irregular, random arrangement of particles with only short-range order, making them isotropic with a range of melting points. Plastic and glass are amorphous solids.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views1 pageUnit 1 Solid State Just Introduction
Unit 1 Solid State Just Introduction
Uploaded by
Jothis JoseThe document discusses the classification of solids into crystalline and amorphous solids. Crystalline solids have a regular, repeating arrangement of particles with long-range order that gives them anisotropic properties and sharp melting points. Examples include NaCl and diamond. In contrast, amorphous solids have an irregular, random arrangement of particles with only short-range order, making them isotropic with a range of melting points. Plastic and glass are amorphous solids.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
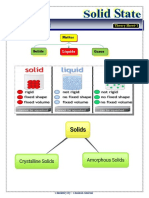
Unit 1 – THE SOLID STATE
Based on the arrangement of constituent particles solids classified into 2 groups.
Classification of solids
Crystalline Solids Amorphous solids
# Regular, repeating, # irregular random manner
alternating manner. (true (Pseudosolids or supercooled
solids) liquids)
# Long range order #Short range order
#Gives regular cleavage on #Gives irregular cleavage when
cutting. we cut
#Anisotropic #Isotropic
#Sharp melting point #has a range melting point
#NaCl, Diamond, Naphthaline #Plastic, rubber, glass etc.
etc.
What is the difference between isotropic and anisotropic nature?
Ans: Anisotropy – Physical properties like electrical resistance or refractive
index show different values when measured along different directions in the
same crystals.
Isotropic – Refractive index of a solid is observed to have the same value along
all directions.
From the following choose the incorrect statement about crystalline solids.
a) Melt at sharp temperature
b) Have long range order
c) They have definite heat of fusion
d) They are isotropic
1015208919 1015208919
You might also like
- 1 Solid State.Document12 pages1 Solid State.ashok pradhanNo ratings yet
- 5921apni KakshaDocument109 pages5921apni KakshaVimal PrasadNo ratings yet
- CRYSTALDocument28 pagesCRYSTALakinlangsigyuuuNo ratings yet
- Lakshya JEE AIR (2025) : Solid StateDocument67 pagesLakshya JEE AIR (2025) : Solid Stateb63672304No ratings yet
- Solidstate Part 1Document35 pagesSolidstate Part 1Gopikrishna ThirunavukarasuNo ratings yet
- SheetDocument108 pagesSheetPinkyNo ratings yet
- Chapter 1-: Introduction To Solid State PhysicsDocument46 pagesChapter 1-: Introduction To Solid State PhysicsAlya RahimNo ratings yet
- CBSE Class 12 Solid State Study NotesDocument414 pagesCBSE Class 12 Solid State Study Notespranav kudesiaNo ratings yet
- Nature of CrystalDocument24 pagesNature of CrystalYeh I'mBreadNo ratings yet
- Chemistry: Class - 12 SubjectDocument7 pagesChemistry: Class - 12 SubjectHemant YadavNo ratings yet
- Solid StateDocument37 pagesSolid StateSai Sasivardhan GampaNo ratings yet
- Solid State Notes Jee Main and Advanced PDFDocument36 pagesSolid State Notes Jee Main and Advanced PDFVedant BiyaniNo ratings yet
- 1a.SOLID STATE (01-25)Document25 pages1a.SOLID STATE (01-25)karansharma690No ratings yet
- Solid StateDocument166 pagesSolid StateSanghamitra ChakrabortyNo ratings yet
- Solid State PDFDocument75 pagesSolid State PDFNishali SamNo ratings yet
- Hbse Revision CapsuleDocument18 pagesHbse Revision CapsuleS.S. Tutorials Radaur OfficialNo ratings yet
- CH-1 (Xii)Document11 pagesCH-1 (Xii)Vijay KumarNo ratings yet
- Solid State Class 1 (4th May 2022) Handout and Home WorkDocument89 pagesSolid State Class 1 (4th May 2022) Handout and Home WorkShivacharan HollaNo ratings yet
- JEE Advanced 2023 Solid State Revision Notes - Free PDF DownloadDocument11 pagesJEE Advanced 2023 Solid State Revision Notes - Free PDF Downloadhishamkalliyath19No ratings yet
- Physical ChemistryDocument5 pagesPhysical ChemistryJothi KNo ratings yet
- By Prin Ces Ir: He Olid TateDocument49 pagesBy Prin Ces Ir: He Olid TateVinayNo ratings yet
- 3.crystalline and Amorphous SolidsDocument28 pages3.crystalline and Amorphous SolidsJohncelle khent BagorioNo ratings yet
- Solid-State 01Document1 pageSolid-State 01javeed akthaar sNo ratings yet
- Sample Chemistry Chapter Without BrandingDocument59 pagesSample Chemistry Chapter Without BrandingNitin JethwaNo ratings yet
- Physics - Unit - 07 (E)Document16 pagesPhysics - Unit - 07 (E)Balaji RamakrishnanNo ratings yet
- Solid StateDocument41 pagesSolid State45 XI A Subham SharmaNo ratings yet
- Types of SolidsDocument39 pagesTypes of SolidsTHARIK ANWAR100% (2)
- Solid StateDocument75 pagesSolid StateChaitanyaPeshin100% (1)
- 2 Cot 1 2023 Crystalline and Amorphous SolidsDocument26 pages2 Cot 1 2023 Crystalline and Amorphous SolidsRG Leeh Tanatan AndrajeNo ratings yet
- 11.5.2020 - Solid States NEET DHOOM Series #1Document88 pages11.5.2020 - Solid States NEET DHOOM Series #1AlokNo ratings yet
- 1.solid StateDocument33 pages1.solid StateBHAVITH SD VNS 06No ratings yet
- The Solid State: Crystalline SolidsDocument3 pagesThe Solid State: Crystalline Solidsmillinagi95No ratings yet
- Solid State - Study Material - Yak9Document33 pagesSolid State - Study Material - Yak9Amrit Kumar BiswasNo ratings yet
- Solid State Chemistry Maharashtra State BoardDocument27 pagesSolid State Chemistry Maharashtra State BoardPankaj JindamNo ratings yet
- Solid State Pharmaceutics: Submitted To-Dr Shishu Submitted by - Tania PawarDocument33 pagesSolid State Pharmaceutics: Submitted To-Dr Shishu Submitted by - Tania Pawarncpharma100% (1)
- Solid StateDocument22 pagesSolid State8446988233bwdNo ratings yet
- Solid State Notes PDFDocument36 pagesSolid State Notes PDFGyanendra Gs100% (1)
- ES Module 3 - Quarter 1 - Types of SolidsDocument13 pagesES Module 3 - Quarter 1 - Types of SolidsAnalynAsuncionAtaydeNo ratings yet
- Types of SolidsDocument14 pagesTypes of SolidsAbubakar KhattarNo ratings yet
- Solids Lecture 01Document14 pagesSolids Lecture 01agha chandioNo ratings yet
- Olid Tate: 1. SolidsDocument1 pageOlid Tate: 1. SolidsHaRryNo ratings yet
- Solid State Class 12 Notes by Furqan Ahmad SirDocument7 pagesSolid State Class 12 Notes by Furqan Ahmad SirANJANA SHARMANo ratings yet
- Noo Xii Ch01 Solid StateDocument51 pagesNoo Xii Ch01 Solid StateG boiNo ratings yet
- SOLID STATE (School)Document36 pagesSOLID STATE (School)CHIRAG VOHRANo ratings yet
- Solid State Chemistry IPEDocument15 pagesSolid State Chemistry IPEAdiChemAdi100% (4)
- Solid State - Lecture 1 - 14-05-2021Document91 pagesSolid State - Lecture 1 - 14-05-2021Pruthvi BNNo ratings yet
- Solid States NotesDocument34 pagesSolid States NotesTNo ratings yet
- Aerospce Batch 3 PHY 4201 Lecture 1Document26 pagesAerospce Batch 3 PHY 4201 Lecture 1Rasel khanNo ratings yet
- Class 12 Chemistry Chapter 1 Solid States (Typed Notes)Document12 pagesClass 12 Chemistry Chapter 1 Solid States (Typed Notes)Shaku JoshiNo ratings yet
- The Solid State: Chapter - 15Document16 pagesThe Solid State: Chapter - 15Athish MNo ratings yet
- Squid Dame PPDocument27 pagesSquid Dame PPJaier DojolesNo ratings yet
- Difference Between Crystalline and Amorphous SolidsDocument24 pagesDifference Between Crystalline and Amorphous SolidsShashwat KhuranaNo ratings yet
- College Notes Unit-1 Solid StateDocument24 pagesCollege Notes Unit-1 Solid StateRamanujam JNo ratings yet
- Solid Crystalline, Amorphous & Polymorphism PDFDocument33 pagesSolid Crystalline, Amorphous & Polymorphism PDFPrabhas Meher100% (1)
- Hsslive XII Quick Notes For Half Yera Exam ElvinDocument23 pagesHsslive XII Quick Notes For Half Yera Exam ElvinRavindra100% (1)
- Lecture 8 Physics I 27.01.22Document95 pagesLecture 8 Physics I 27.01.22indulal lalNo ratings yet
- Solid StateDocument25 pagesSolid StateDigvijay SolankiNo ratings yet
- LP - GC - Nature of SolidsDocument9 pagesLP - GC - Nature of SolidsAllyza SobosoboNo ratings yet
- Dichroscopes Made Easy: The "RIGHT-WAY" Guide to Using Gem Identification ToolsFrom EverandDichroscopes Made Easy: The "RIGHT-WAY" Guide to Using Gem Identification ToolsRating: 5 out of 5 stars5/5 (2)
- Crystal Harmony: healing properties of crystals and how to use them to harmonise your lifeFrom EverandCrystal Harmony: healing properties of crystals and how to use them to harmonise your lifeNo ratings yet