Professional Documents
Culture Documents
CPP Electrochemistry
Uploaded by
Naman MishraOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CPP Electrochemistry
Uploaded by
Naman MishraCopyright:
Available Formats
Solved Example
Zn (s) has Kx1000 _ 2.48x10x1000
Ex.1 The reaction :Zn2 (aq) +
2e a
Sol. (A) Am
electrode potential of-0.76 V. This means- M 0.20
(A) Zn cannot replace hydrogen from acids = 1.24 ohm- cm* mol-l
(B) Zn is reducing agent
(C) Zn is oxidizing agent Ex.5 When an electric current is passed throuoh
(D) Zn2* is a reducing agent acidulated, water, 112 mL of hydrogen pas
that Zn2* is N.T.P. collects at the cathode in 965
Sol. (B) Negative electrode potential shows seconds
difficult to be reduced and therefore, Zn acts as The current passed, in amperes, is-
reducing agent. (A) 1.0 (B) 0.5
Ex.2 Other things being the same, the Ecell of the (D) 2.0
(C)0.1
Daniel cell may be increased by. Sol. (A) 22,400 mL of hydrogen at STP(or NTP) =2g
(A) Keeping low temperature
. 112 mL of hydrogen at
(B) Using large copper electrode
(C)Usingsmall zinc electrode STP 2gx112ml
22,400mL
=
10-2 g
(D) Decreasing the conc. of Cu2
Sol. (A) Zn (s) +Cu2+ (aq)-> Zn2* (aq) + Cu (s)
2Ht +2e>H2
Fcellcll 2.303 RT oo Zn*] 2F 1 mol
nF [Cu2]
Decrease in temperature makes the value of
=2 x
96,500C=2g
2g hydrogen is deposited by 2 x 96,500 C
2.303 RT
smaller. Smaller the value of this 1 0 g hydrogen will be deposited by
nF
factor, greater is Ecell
2x96,500x10"g =965 C
2g
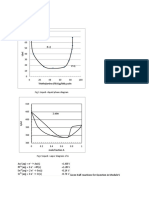
Ex.3 The equivalent conductivities at infinite dilution
of the cation and the anion of a salt A,B are 140 Q=ixt
and 80 ohm cm2 eq respectively. The 965 i x 965
equivalent conductivity of the salt at infinite i= 1
dilutionis
(A) 160 ohml cm2 eql
40.5 g of Al
(B) 220 ohm"l cmé eq" Ex.6 The charge required to deposit
(atomic mass = 27.0 g) from the fused A
(C)60 ohml cm2 eq-i
(D) 360 ohml cm2 eq-l (SO) is
Sol(D) A (A4B) = * (At)+ g (B) (A) 4.34 x 10 C (B) 43.4 x 10 C
140+80=220 ohm cm2 eq (C) 1.44x 105 (D) None of these
Ex.4 The specific conductance of Sol. (A) A3+ +3e- A l
a 0.20 mol L
solution of an electrolyte at 20°C is 2.48 x 10 3F 1 mol 27.0 g 500C
ohm- cm, The molar conductivity of the to deposite 27g required charge = 3 x96,300
solution is -
(A) 1.24 ohm-l cm2 mo-1 . to deposite 40.4g required charge
(B) 4.96 ohm- cm2 mo 40.5gx 3molx 96,500C mol
C)1.24 ohm-l cm2 27.0g
(D) 4.96 ohm-! cm2
=
4.34x 105 C
same amount of electricity was passed
Ex.9 Three
The
through
two separate electrolytic cells faraday of electricity is
passed through
Ex. containing solutions of nickel nitrate and aqueous solutions of
AgNO3, NiSO4 and
nium nitrate respectively. f0.3 g of nickel
c h r o m i u r
kept in three vessels using inert electrodes.CrCl
The
ratio in mol in which the
was
deposited in the first cell, the amount of metals Ag, Ni and Cr
deposited is (At. wt. Ni will be deposited is-
chromiur 59, Cr
=
=
52)
(A)1:2:3 (B)3:2:1
(A)0.1g (B) 0.176 g (C)6:3:2 D) 2:3:6
(C) 0.3 g (D) 0.6 g
mass of Ni
Sol (C) ) Agt(ag) + er A g (s)
mNi = Eq.
Sol (B) UNM I mol = IF
of Cr I mol
m Eq. mass
3F 3 mol
For Ni2t and Crst, we have (ii) Ni2*(aq) +2e N i (s)
0.3g 59/2 2 mol 2 F I mol
mcr 52/3 3F 3/2 mol
52 (ii) Cr3 (aq) +3e>Cr(s)
0.3gx 3 mol 3F I mol
mCr =0.176 g
(59/2) The required ratio of moles of Ag, Ni and Cr is
3 mol Ag:3/2 mol Ni: 1 mol Cr
Electrolytic conduction differs from metallic 6 mol Ag : 3 mol Ni: 2 mol Cr.
Ex.8 or
conduction. In case of metallic conduction -
(A) The resistance increases with increasing Ex.10 In the reaction
temperature 4 Fe +30, > 4 Fe3+ +602-
(B) The resistance decreases with increasing is correct
which.of the following statements
temperature
heat (A) A redox reaction
(C) The flow of currnet does not generate
of the length (B) O is reducing agent
(D) The resistance is independent
of electrolytic conductor (C) Fe3t is an oxidizing agent
vibration of Kernal (D) Fe is reduced to Fe3+
Sol. (A) With increase in temperature oxidized to Fe3t and O, is
therefore, conduction
(Cation) increases and Sol. (A) In this reaction, Fe is
of the metallic
decreases and hence, resistance reduced to 02
conductor increases.
ucstons Electrolysis Q.9 If mercury is used as cathode in the electrolysis
of aqueous NaCl solution, the ions discharged
at cathode are-
Q.1 The passage of current through a solution of
certain (A)H (B) Na
electrolye results in the evolution of H2 (C) OH (D) C
at cathode and Cl at anode. The electrolytic
solution is -
Pesttons Electrolytic conductance
(A) Water (B) HSO based.on.
*** *************
(C) Aq. NaCI (D) Aq. CuClh
Q.10 The specific conductance of a solution is 0.3 568
Q.2 In an electrolytic cell current flows from- ohm cm'. When placed in a cell the
(A) Cathode to anode in outer circuit conductance is 0.0268 ohm'. The cell constant
(B) Anode to cathode outside the cell iS-
(C) Cathode to anode inside the cell (A) 1.331 cm (B) 13.31 cm
(D) Anode to cathode inside the cell (C) 0.665 cm (D) 6.65 cm
Q.3 When an aqueous solution of HS04 is Q.11 A conductance cell was filled with a 0.02 M
electrolysed, the ion discharged at anode is - KCI solution which has a specific conductance
(A) H of 2.768 x 10 ohm cm, If its resistance is
(B) OH
(C) SO (D) 02 82.4 ohm at 25°C, the cell constant is-
(A) 0.2182 cm (B) 0.2281 cm
Q.4 1 mole of Al is deposited by X coulomb of (C) 0.2821 cm (D) 0.2381 cm
electricity passing through aluminium nitrate The variation of equivalent conductance vs
solution. The number of moles of silver Q.12
decrease in concentration of a strong electrolyte
deposited by X coulomb of electricity from is correctly given in the plot -
silver nitrate solution is -
(A) 3 (B)4
(C) 2 (D) 1 Acq
(A) (B)
Q.5 A solution of Na2S04 in water is electrolysed
1/N>
1/N-
Pt the cathode
using clectrodes.The products
and anode are respectively -
at
(A) H2, S02 (B) O2, NaOH
(C) H2, O2 (D) O, SO;
(C) (D)
Q.6 In electrolysis of a fused salt, the weight
deposited on an electrode will not depend on- 1/N- 1/N
(A) Temperature Q.13 Which of the
(B) Current intensity following solutions has the highest
(C) Electrochemical equivalent of ions equivalent conductance?
(A) 0.01M NaCl (B) 0.050 M NaC!
(D) Time for electrolysis
(C) 0.005M NaCI (D) 0.02M NaCl
Q.7 Which loses charge at cathode
Q.14 The resistance of 0.01N solution of an
(A) lons electrolyte AB at 328K is 100 ohm. The
(B) Cations specific conductance of solution is (cell
(C) Anions constant= 1cm")-
(D) Both anions and cations (A) 1000hm (B) 10ohm
Q.8 In the electrolysis of CuSO, the reaction (C) 10ohm cm (D) 10* ohm-cm
Cu+2e>Cu, takes place at Q.15 For an electrolytic solution of 0.05 mol L', the
(A) Anode (B) Cathode conductivity has been found to be 0.0110 Sem.
(C) In solution (D) None
The molar conductivity is
(A) 0.055 S cm mol (B) 550 Scm mor
(C)0.22 S cm mol (D) 220 Scm mol
Two electrodes are fitted in conductance cell 1.5
Q.16 Q.24 The number of
cm apart while the area of cross section of each faraday required to generate
I mole
electrode is 0.75 cm. The cell constant is ofMg from MgClh is-
(A) 1
(A) 1.125 (B) 0.5 cm (B) 2
(C) 3
(C) 2.0 cm (D)0.2 cm (D) 4
Q.25 What weight of copper (At.mass 63.5) deposits
The best conductor of electricity is in IM
Q.17 when 2Faraday of
solution of clcctricity is passed through
cupric salt-
(A) C1,COOH (B) H,SO (A) 63.5g
(CHPO (D)Boric acid (B) 31.75g
(C) 127g (D) 2.0g
on araday's Law of Electrolysis
www.
Q.26 How many coulombs of
electricity are required
for the oxidation of lmole
A certain
current liberates 0.504 of H,0 to O2.
Q.18 g of H2 in (A) 9.65 x 10°C
(B)4.825x 10'C
2 hours. How many grams of copper can be C) 1.93x 10°C (D) 1.93 x 10C
liberated by the same current flowing for
sametime in CuSO, solution-
the
Q.27 How long 2.5amp of current is passed to supply
(A) 31.8 g (B) 16.0 g 54000 C of charge-
(C)12.7g (D) 63.5 g (A) Ihr (B) 2.5hr
(C) 6hr (D) 9hr
Q.19 A current of 2.6 ampere is
passed through
CuSO solution for 6 minutes 20 seconds. The Q.28 1 Faraday of electricity will liberate 1 mole of
amount of Cu deposited is (At. wt. of Cu = the metal from the solution of
63.5, Faraday = 96500 C)-
(A) Copper sulphate (B) Calcium chloride
(A) 6.35 g (B) 0.635 g (C) Gold (1) Chloride (D) Silver () Chloride
(C) 0.325 g (D) 3.1755g
Q.29 Charge in coulombs is equal to-
Q.20 Three Faradays of electricity are passed
through (A)Faraday
molten Al,03, aqueous solution of CuSO4 and
av. number
molten NaCl taken in three different electrolytic
(B) Faraday x av. number
cells. The amount of AI, Cu and Na deposited at
the cathodes will be in the ratio of av.number
C)
(A) I mole 2 mole:3 mole Faraday
(B) I mole: 1.5 mole:3 mole (D) None of these
(C) 3 mole: 2 mole: I mole
(D) I mole : 1.5 mole : 2 mole Q.30 A current of 2
ampere was passed through
solutions of CuSO, and AgNO, in series. 0.635 g
of copper was deposited. Then the
Q.21 The quantity of electricity required to liberate weight of
silver deposited will be-
0.01g equivalent of an element at the electrode
is
(A) 0.59 g (B) 3.24 g
(C) 1.08 g D) 2.16 g
(A) 9650C (B) 96500Cc
(C)965C (D) 96.5C Q.31 On passing 3ampere of electricity for
50 minutes, 1.8g of metal deposits. The
Q.22 The unit of electrochemical
equivalent is- equivalent mass of metal is-
(A) gm ampere (B) gm/coulomb (A) 20.5 (B) 25.8
(C) gm-ampere (D) coulomb/gram (C) 19.3 (D) 30.7
Q.23 One faraday of
electricity will liberate one mole Q.32 The number of coulombs required to deposit
of metal from a 5.4g of Al when the electrode reaction is-
solution of
(A) AuCl (B) CuSO A+3e>Al
(C) BaCl (D) KCI (A) 1.83x 103c (B) 57900C
(C) 5.86x 10®C (D) None of the above
Ouestigns Electro chemical series and Cuestions Emf of the cell
busel on
bHsed on Electrode potential
Q.39 Which of the following will increase the voltage
The rcaction 1/2 H2 (g) + AgCl (s) - H' (uq) + of the cell
Q.33
CF (aq)+ Ag ($) can be represented in the Sn(s) +2Ag (aq)> Sn" (aq)) +2 Ag (s)
galvanic cell as- (A) Increase in the concentration of Sn2t ions
(A) Ag lAgCI (S)} KCI (sol) |JAgNO,(sol)}Ag (B) Increase in the concentration of Agt ions
(B) PtH (g) | HCI (sol)||AgNO, (sol) (C) Increase in the size of silver rod
(C) PJH; (g)| HCI (sol) ||AgCI ()| Ag (D) None
(D) PH: (g)|KCI (sol)||AgCl ($) Ag
At 298 K, the standard reduction potentials for Q.40 The standard oxidation potentials, E° for the half
Q.34
the following half reactions are given as reactions are as Zn Zn° + 2e, E° 0.76 V
=
=
Zn (aq) + 2 e > Zn (s); - 0.762 Fe Fe2t+ 2e, E° =
0.41 V. The emf for the
cell reaction Fes" + Zn > Zn*" +Fe, is -
Cr (aq)+ 3e>Cr(s); -0.740 (A)-0.355V (B) +0.35 V
2H (aq)+ 2e H2 (g); 0.00 (C) +1.17 V (D)-1.17 V
Fe3t (aq) + e > Fe4t (aq); + 0.770
The strongest reducing agent is - Q.41 The single electrode potential E of 0.1 M
(A) Zn(s) (B) H2(g) solution ofM" ions [E°R=-2.36 V] is -
(C) Crs) (D) Fe2t(ag) (A)+2.41 (B)-2.41
(C)-4.82 (D)+4.82
Q.35 The standard electrode potential of Zn, Ag and
Cu are 0.76, 0.80 and 0.34 volt respectively; Q.42 E values of Mg" IMg, Fe"| Fe and Zn*| Zn are
then 2.37 V, 0.44 V and 0.76 V respectively.
- -
The correct statement is -
(A) Ag can oxidise Zn and Cu
(A) Mg oxidises Fe (B) Zn oxidises Fe
(B) Ag can reduce Zn2t and Cu2+ (C) Zn reduces Mg D) Zn reduces Fe
(C) Zn can reduce Agt and Cu2+
(D) Cu can oxidise Zn and Ag Q43 For the reactions
Q.36 An aqueous solution of CuSO4 is stirred with a MnO4+8Ht+5e Mn +4H0,
E 1.51 V
silver spoon. The following will happen-
MnO2 +4H +2e Mn" +2H20,
(A) Cut will be formed
E° 1.23V then for the reaction
(B) Agt will be formed
(C) Cu will be precipitated Mn04+4H*+ 3e> MnO2 +2H20, E°is
(D) Nothing will happen (A) 1.70 V (B) 5.09 V
Q.37 Consider following half-cell reaction- (C) 0.28 V (D) 0.84 VN
E = 0.96 V
.Ate+A" Q.44 Consider the following equations for a cell
II. B+eB2- E°-0.12 V reaction
IIL C+e+C E =+0.18V A+B C+D E°=x volt, Keg=K|
IV. D2t+ 2e D E -1.12 V 2A +2B 2C+2DE° =
y volt, Ka =K2
What combination of two half-cells would then
result in a cell with the largest potential? (B)x-2y, K =2K2
(A) I and II (B) I and III
(A)x=y,K=K2
C)I and IV (D) II and IV (C)x=y,K12-K2 (D)x=y. K2-K2
Q45 Standard electrode potentials of
Q.38 StandardE of the half cell Fe | Fet is +0.44V
Fe2t+2e Fe and Fe3t+3e Fe
standard E of half cell Cu | Cu2t is Th
0.32V, then-
are 0.440 V and 0.036V respectively.
standard electrode potential (E) for
(A) Cu oxidises Fe2t ion (B) Cu2t oxidises Fe
Fe3t + Fe2t is -
(C) Cu reduces Fe2t ion (D) Cu2t reduces Fe (A)-0.476 V (B)-0.404 V
(C) +0.404 V (D) +0.772V
At equilibrium - Q.51 The value of the reaction quotient, Q, for the cell
Q.46 AG° =
0 (B) Ecell =0, AG= 0
(A) E cell=0, Zn(s)Zn2*(0.01 M)||Ag*(1.25 M)\Ag(s) is-
both are correct (D) none is correct (A) 156 (B) 125
(C)
(C) 1.25 x 10-2 (D) 6.40 x 10-3
Q.47 emfof cell Ni | Ni<t (1.0M) || Au3* (1.0M)] Au
f E for Ni4| Ni is 0.25 V, EO for
-
is uestions Tvnes of cell & corrosion
Au| Au is 1.50V- based on
(A) + 1.25 V (B)-1.75V
(C)+1.75V (D) +4.0V The Zn acts as sacrificial or cathodic protection
Q.52
to prevent rusting ofiron because
Normal AI - AICl3 coupled with standard
Q.48
hydrogen electrode gives an emf of 1.66V. The (A) EOP of Zn < E°OP 0f Fe
standard oxidation electrode potential of (B) EOP 0f Zn > EOP of Fe
aluminium is-
(A)- 1.66V (B) +1.66V (C) EOP 0f Zn =
E°OPp of Fe
(D) Zn is cheaper than iron
(C)-0.83V (D)+0.833V
The cell reaction Zn +Cu~t Zn2* +Cu, is Q.53 In electrochemical corrosion of metals, the
Q.49
best represented by -
metal undergoing corrosion
(A) Cu/Cu2|| Zn2+/Zn (A) Acts as anode
(B) Acts as cathode
(B) Zn/Zn2 || Cu2t/ Cu (C) Undergoes reduction
(C) Cut/Cu|| Zn/ Zn2+ (D) None
(D) Pt/Zn2*|| Pt/ Cu2+
Q.54 When a lead storage battery is charged it acts
Consider a voltaic cell based on these half-cells as
Q.50
+0.80 V (A) A fuel cell
Agt (aq)+ - Ag(s); E°
=
E° =-0.40 V
(B) An electrolytic cell
Cd (aq)+2e Cd(s); (C) A galvanic cell
of the
Identify the anode and give the voltage (D) A concentration cell
cell under standard conditions
-
(B) Ag: Ecell 2.00V
=
(A) Ag: Ecell 0.40 V
C)Cd; Ecll =
1.20 V (D) Cd; Ecell=2.00V
8Vaa aa a|3|va l a Suy
6 8t L 9t St I ONÒ
S tS£S TSIS|os
ESZS tt E
a a3|v |a |avao v aa o suy
suy
Ore8Le9EsEPEEEzEIE0E6S49 ST t z z 7 1 N O
V
Suy
07
61 S LI9s ETII or68 ON'O
You might also like
- Check List For Site Construction WorksDocument65 pagesCheck List For Site Construction WorksUmar Farooq91% (11)
- Experiment 7-Atterberg LimitsDocument14 pagesExperiment 7-Atterberg LimitsJack RasalNo ratings yet
- Final Research Paper of Group 3Document36 pagesFinal Research Paper of Group 3mondejar loueljayjustoNo ratings yet
- ElectrochemistryDocument17 pagesElectrochemistryzohaibsalamNo ratings yet
- Fundamentals of Automatic Control For Building SystemsDocument200 pagesFundamentals of Automatic Control For Building SystemstrungNo ratings yet
- Chapter 3 ElectrochemistryDocument8 pagesChapter 3 Electrochemistrymeshal retteryNo ratings yet
- New ThermoformingDocument20 pagesNew ThermoformingVijay S PNo ratings yet
- Handbook of PolyblendsDocument2,529 pagesHandbook of PolyblendsSoumava PalitNo ratings yet
- Practice Exam 4Document7 pagesPractice Exam 4Hasantha PereraNo ratings yet
- Exercise - I: (Only One Option Is Correct)Document3 pagesExercise - I: (Only One Option Is Correct)Amudala HemashviniNo ratings yet
- Day-5 - In-Class Assignment - : Phase-1Document4 pagesDay-5 - In-Class Assignment - : Phase-1Arnab DasNo ratings yet
- Subject: Chemistry Electrochemistry: Decreases PH of Solution (D) Electrolysis of CusoDocument28 pagesSubject: Chemistry Electrochemistry: Decreases PH of Solution (D) Electrolysis of CusoQwertyNo ratings yet
- SAQ Ans 20Document4 pagesSAQ Ans 20Mujtaba RashadNo ratings yet
- Electrochemistry: WWW - Crackjee.xyzDocument8 pagesElectrochemistry: WWW - Crackjee.xyzRashmi Ranjan DasNo ratings yet
- Electrochemistry: 0 8 0 79 0 34 2 37 Ag / Ag - HG / HG - Cu / Cu - MG / MGDocument11 pagesElectrochemistry: 0 8 0 79 0 34 2 37 Ag / Ag - HG / HG - Cu / Cu - MG / MGAnikin Skywalker100% (1)
- Xi Centre Che 18.03.24Document16 pagesXi Centre Che 18.03.24pinnaacleclasses salemNo ratings yet
- Answer Key (CH - Electrochemical Set-1) 12thDocument12 pagesAnswer Key (CH - Electrochemical Set-1) 12thAgrim TanejaNo ratings yet
- CHEMISTRY BOOK-3 OBJECTIVE QUESTIONS SOLUTIONS ELECTROCHEMISTRYDocument8 pagesCHEMISTRY BOOK-3 OBJECTIVE QUESTIONS SOLUTIONS ELECTROCHEMISTRYwaliasanchit007No ratings yet
- ELECTROCHEMISTRY REVIEWDocument8 pagesELECTROCHEMISTRY REVIEWAshwin Balaji100% (1)
- H011201080 - Nuralifa Rezky Mustika - Tugas Individu5Document5 pagesH011201080 - Nuralifa Rezky Mustika - Tugas Individu5Nuralifa Rezky MustikaNo ratings yet
- ElectrochemistryDocument21 pagesElectrochemistrypremathangam807No ratings yet
- 12 Chemistry Chapter 3 Assignment 5Document2 pages12 Chemistry Chapter 3 Assignment 5sansharmajsNo ratings yet
- Electrochemistry - DPP 04Document2 pagesElectrochemistry - DPP 04Lakshya FalorNo ratings yet
- Physical-Chemistry ElectrochemistryDocument10 pagesPhysical-Chemistry ElectrochemistryHarshad SSNo ratings yet
- N2016 H2 P1 Solns for UploadingDocument8 pagesN2016 H2 P1 Solns for UploadingjkNo ratings yet
- CH 17Document42 pagesCH 17Bông Cải XanhNo ratings yet
- Electrochemistry - PYQ - (NSEC)Document5 pagesElectrochemistry - PYQ - (NSEC)LAKHAN KHANDELWAL100% (1)
- Solutions To Problem Set 2Document5 pagesSolutions To Problem Set 2Andy Nguyen100% (1)
- Physical ChemistryDocument1 pagePhysical ChemistryEnsemble StarsNo ratings yet
- LT Iit Che DPT - 15 - 21.02.2024Document3 pagesLT Iit Che DPT - 15 - 21.02.2024Deena chemistNo ratings yet
- Homework Packet Unit 9 AnswersDocument3 pagesHomework Packet Unit 9 AnswersHelloNo ratings yet
- Electrochemical ThermodynamicsDocument38 pagesElectrochemical ThermodynamicsikamelyaastutiNo ratings yet
- ElectrochemistryDocument4 pagesElectrochemistryTwisha ViraniNo ratings yet
- Topic 9 (Galvanic Cell) - Tutorial - Level 1 AnswerDocument5 pagesTopic 9 (Galvanic Cell) - Tutorial - Level 1 AnswerCheng Xun LeeNo ratings yet
- 2 MS ElectrochemistryDocument7 pages2 MS ElectrochemistrysachinNo ratings yet
- When A Non-Spontaneous Redox Reaction Is Made To Occur by Putting Electrical Energy Into The SystemDocument11 pagesWhen A Non-Spontaneous Redox Reaction Is Made To Occur by Putting Electrical Energy Into The SystemArdit QerimiNo ratings yet
- ElectrochemistyDocument21 pagesElectrochemistyAagash PranavNo ratings yet
- Class 12 Chemistry Ch-2.ElectrochemistryDocument37 pagesClass 12 Chemistry Ch-2.Electrochemistrykarnan karupiahNo ratings yet
- 6433 Topper 21 129 510 2 43 Electrochemistry Up201612091847 1481289429 3Document44 pages6433 Topper 21 129 510 2 43 Electrochemistry Up201612091847 1481289429 3Rishab PurkayasthaNo ratings yet
- Retest Answer Key 29 JanDocument5 pagesRetest Answer Key 29 Jangoddhruv17No ratings yet
- ChemDocument10 pagesChemAnshika singh sisodiyaNo ratings yet
- 01 - Electro Chemistry (Level) Module-6-1Document16 pages01 - Electro Chemistry (Level) Module-6-1Raju SinghNo ratings yet
- C - 5 A - 2 (Electrochemistry)Document10 pagesC - 5 A - 2 (Electrochemistry)Steven GuptaNo ratings yet
- Practice Problems 7Document15 pagesPractice Problems 7Deena RuangchayNo ratings yet
- ElectroDocument9 pagesElectromoin19usmanNo ratings yet
- ELECTROCHEMISTRYDocument12 pagesELECTROCHEMISTRYChangha ParkNo ratings yet
- Xicbse Electrochemistry-Asst 3 AnsDocument2 pagesXicbse Electrochemistry-Asst 3 Anskavidivikannan2005No ratings yet
- Lab Report: Cmt555: Experiment 1: Galvanic & Electrolytic CellDocument11 pagesLab Report: Cmt555: Experiment 1: Galvanic & Electrolytic CellkuekNo ratings yet
- Chapter 24 Reactions in Chemical Cells (Extension)Document3 pagesChapter 24 Reactions in Chemical Cells (Extension)sliversniperNo ratings yet
- Homework 1 SolutionsDocument18 pagesHomework 1 SolutionsThomas HoNo ratings yet
- Module 7 Problem Set Answer KeyDocument3 pagesModule 7 Problem Set Answer KeyPauline Grace CadusaleNo ratings yet
- ElectrochemistryDocument3 pagesElectrochemistryArchanaa PadmavathiNo ratings yet
- Day - I: Solved Objective Examples: Example 1Document11 pagesDay - I: Solved Objective Examples: Example 1Rahul SinghNo ratings yet
- Electrochemistry - Workbook SolutionDocument41 pagesElectrochemistry - Workbook SolutionSamNo ratings yet
- CH 17Document43 pagesCH 17ዝምታ ውስጤ ነውNo ratings yet
- ELECTROCHEMISTRYDocument2 pagesELECTROCHEMISTRYShivaanee SKNo ratings yet
- Electrochemistry worksheet covering EMF of cells, standard electrode potentials, electrolysis, Faraday's lawDocument2 pagesElectrochemistry worksheet covering EMF of cells, standard electrode potentials, electrolysis, Faraday's lawpankaj singhNo ratings yet
- Adobe Scan 24-Feb-2024Document16 pagesAdobe Scan 24-Feb-2024Rudra SinghNo ratings yet
- Electrochemistry: Practice QuestionsDocument10 pagesElectrochemistry: Practice Questionsshikha nathNo ratings yet
- MHT Cet 20221662555106Document7 pagesMHT Cet 20221662555106Some random nameNo ratings yet
- Practice Electrochemistry - 1Document5 pagesPractice Electrochemistry - 1ervaldiNo ratings yet
- 3.electrochemistry KCET PYQsDocument2 pages3.electrochemistry KCET PYQsPunith kumar50% (2)
- MR 477 ElectrometallurgyDocument2 pagesMR 477 ElectrometallurgyChelseaNo ratings yet
- infinIITy-12_10-04-24_StudentDocument2 pagesinfinIITy-12_10-04-24_StudentshouryatrialNo ratings yet
- Electrochemistry and its applicationsDocument39 pagesElectrochemistry and its applicationsHaider AliNo ratings yet
- Electrochemistry Mittal Sir: Worksheet-I Objective QuestionsDocument3 pagesElectrochemistry Mittal Sir: Worksheet-I Objective QuestionstarunNo ratings yet
- IntroductionDocument31 pagesIntroductionAhmed FaragallahNo ratings yet
- Boyle's and Charles's Laws WorksheetDocument3 pagesBoyle's and Charles's Laws WorksheetARVIN CONCHANo ratings yet
- Chapter-i-Introduction To Soil MechanicsDocument27 pagesChapter-i-Introduction To Soil MechanicsVijaykumar Nagnaik100% (2)
- 00.04 Questionnaire Chain ConveyorDocument1 page00.04 Questionnaire Chain ConveyorSandy DumisaniNo ratings yet
- Leak Test Report for Tank 25Document7 pagesLeak Test Report for Tank 25Sibgathullah MohammedNo ratings yet
- Part 4Document52 pagesPart 4Martha ArgerichNo ratings yet
- PomcDocument8 pagesPomcAnirudh AcharyaNo ratings yet
- Sodium Cholate Micelle Formation & Solubilization of Organic CompoundsDocument12 pagesSodium Cholate Micelle Formation & Solubilization of Organic Compoundscs1900No ratings yet
- Cre EquationsDocument6 pagesCre Equationslily augustNo ratings yet
- EC105Document16 pagesEC105api-3853441No ratings yet
- Sigmarine 28 Multi-Purpose Primer Data SheetDocument3 pagesSigmarine 28 Multi-Purpose Primer Data SheetaangNo ratings yet
- Potassium Nitrate Without Anticaking (BP, Ph. Eur.) Pure, Pharma GradeDocument1 pagePotassium Nitrate Without Anticaking (BP, Ph. Eur.) Pure, Pharma GradeMiguel CruzNo ratings yet
- IIT JEE - Mains Model Test Paper - 1 (Physics, Chemistry, Maths)Document12 pagesIIT JEE - Mains Model Test Paper - 1 (Physics, Chemistry, Maths)studysteps.in83% (6)
- Table of PKa and PI ValuesDocument12 pagesTable of PKa and PI ValuesMariel Defensor ContiNo ratings yet
- BM520Document2 pagesBM520semoyapaNo ratings yet
- Paclitaxelhandbookoninjectabledrugs 15thed 160122072333Document9 pagesPaclitaxelhandbookoninjectabledrugs 15thed 160122072333Tami OvetayNo ratings yet
- Applications of BiosensorsDocument7 pagesApplications of Biosensorspradeep kumarNo ratings yet
- D 6917 - 03Document2 pagesD 6917 - 03luis-12No ratings yet
- Physics 2006 P2-2 PDFDocument12 pagesPhysics 2006 P2-2 PDFlalNo ratings yet
- BBR Post-Tensioning en Rev1 0510Document16 pagesBBR Post-Tensioning en Rev1 0510jasamnajNo ratings yet
- 5 Topic 2 Worksheet 5 Photoelectron Spectroscopy STDocument4 pages5 Topic 2 Worksheet 5 Photoelectron Spectroscopy STrudywahudiNo ratings yet
- Rate Analysis 2078-079Document131 pagesRate Analysis 2078-079Pawan PandeyNo ratings yet
- Biology 1010 General ObjectivesDocument2 pagesBiology 1010 General Objectivesapi-241247043No ratings yet