Professional Documents
Culture Documents
Table 2.2: 25 A Listing of The Expected Electron Configurations For Some of The Common Elements
Uploaded by
SAUDOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Table 2.2: 25 A Listing of The Expected Electron Configurations For Some of The Common Elements
Uploaded by
SAUDCopyright:
Available Formats
JWCL187_ch02_018-043.
qxd 11/5/09 8:06 AM Page 25
2.3 Electrons in Atoms • 25
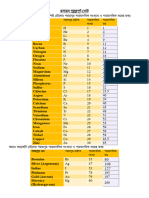
Table 2.2 A Listing of the Expected Electron Configurations for
Some of the Common Elementsa
Atomic
Element Symbol Number Electron Configuration
Hydrogen H 1 1s1
Helium He 2 1s2
Lithium Li 3 1s22s1
Beryllium Be 4 1s22s2
Boron B 5 1s22s22p1
Carbon C 6 1s22s22p2
Nitrogen N 7 1s22s22p3
Oxygen O 8 1s22s22p4
Fluorine F 9 1s22s22p5
Neon Ne 10 1s22s22p6
Sodium Na 11 1s22s22p63s1
Magnesium Mg 12 1s22s22p63s2
Aluminum Al 13 1s22s22p63s23p1
Silicon Si 14 1s22s22p63s23p2
Phosphorus P 15 1s22s22p63s23p3
Sulfur S 16 1s22s22p63s23p4
Chlorine Cl 17 1s22s22p63s23p5
Argon Ar 18 1s22s22p63s23p6
Potassium K 19 1s22s22p63s23p64s1
Calcium Ca 20 1s22s22p63s23p64s2
Scandium Sc 21 1s22s22p63s23p63d14s2
Titanium Ti 22 1s22s22p63s23p63d24s2
Vanadium V 23 1s22s22p63s23p63d34s2
Chromium Cr 24 1s22s22p63s23p63d54s1
Manganese Mn 25 1s22s22p63s23p63d54s2
Iron Fe 26 1s22s22p63s23p63d64s2
Cobalt Co 27 1s22s22p63s23p63d74s2
Nickel Ni 28 1s22s22p63s23p63d84s2
Copper Cu 29 1s22s22p63s23p63d104s1
Zinc Zn 30 1s22s22p63s23p63d104s2
Gallium Ga 31 1s22s22p63s23p63d104s24p1
Germanium Ge 32 1s22s22p63s23p63d104s24p2
Arsenic As 33 1s22s22p63s23p63d104s24p3
Selenium Se 34 1s22s22p63s23p63d104s24p4
Bromine Br 35 1s22s22p63s23p63d104s24p5
Krypton Kr 36 1s22s22p63s23p63d104s24p6
a
When some elements covalently bond, they form sp hybrid bonds. This is espe-
cially true for C, Si, and Ge.
hydrogen, helium, and sodium are, respectively, 1s1, 1s2, and 1s22s22p63s1. Electron
configurations for some of the more common elements are listed in Table 2.2.
At this point, comments regarding these electron configurations are necessary.
valence electron First, the valence electrons are those that occupy the outermost shell. These elec-
trons are extremely important; as will be seen, they participate in the bonding be-
tween atoms to form atomic and molecular aggregates. Furthermore, many of the
physical and chemical properties of solids are based on these valence electrons.
In addition, some atoms have what are termed stable electron configurations;
that is, the states within the outermost or valence electron shell are completely
You might also like
- Electronic Configuration of ElementsDocument3 pagesElectronic Configuration of ElementsVeeresh Ananda Butti88% (8)
- Atoms and Ions Worksheet AnswersDocument1 pageAtoms and Ions Worksheet AnswersFrancis Olila0% (1)
- Atomic Mass and Atomic Number Worksheet KeyDocument1 pageAtomic Mass and Atomic Number Worksheet KeyRalphNacis0% (1)
- GCE Chemistry Data Booklet Issue 2Document35 pagesGCE Chemistry Data Booklet Issue 2purityplus89% (9)
- BS en Iso 1833-12-2010Document12 pagesBS en Iso 1833-12-2010EmkFataAliraqNo ratings yet
- A Listing of The Expected Electron Configurations ForDocument1 pageA Listing of The Expected Electron Configurations ForLemuel ToribioNo ratings yet
- Kala Dan KumpulanDocument2 pagesKala Dan KumpulanSains Pismp 17No ratings yet
- Electronconfigurationforallelements 110227223014 Phpapp01Document2 pagesElectronconfigurationforallelements 110227223014 Phpapp01Anusia ThevendaranNo ratings yet
- Electron Distribution Per OrbitDocument3 pagesElectron Distribution Per OrbitJaswinder BehlNo ratings yet
- All About The Periodic Table - Home Laboratory WorksheetDocument4 pagesAll About The Periodic Table - Home Laboratory WorksheetFrank Ed SerranoNo ratings yet
- Adobe Scan 22-Nov-2020Document1 pageAdobe Scan 22-Nov-2020Priyanshu KumarNo ratings yet
- Class: M3 Subject: Chemistry Chapter 1: Basic Concepts of ChemistryDocument6 pagesClass: M3 Subject: Chemistry Chapter 1: Basic Concepts of Chemistrysamarth chawlaNo ratings yet
- 2.5 Transition Metals: Complex FormationDocument11 pages2.5 Transition Metals: Complex FormationSONIEH SYLVIUSNo ratings yet
- A Periodic TableDocument1 pageA Periodic Tabletownsenr94No ratings yet
- Periodic TableDocument2 pagesPeriodic TableaishterumegumiiichannnNo ratings yet
- Transition ElementsDocument3 pagesTransition ElementsKel FelixNo ratings yet
- Atomic Mass and Atomic Number WorksheetDocument1 pageAtomic Mass and Atomic Number WorksheetGuayNo ratings yet
- 2.5 Revision Guide Transition Metals AqaDocument11 pages2.5 Revision Guide Transition Metals Aqashafiqur rahmanNo ratings yet
- Chemistry Basic Notes For Class 10-11-12 StudentsDocument2 pagesChemistry Basic Notes For Class 10-11-12 Studentshsccrash096No ratings yet
- 5 General Principles of MetallurgyDocument17 pages5 General Principles of MetallurgyChamanthi SagarNo ratings yet
- Assignment For Topic B - 1Document4 pagesAssignment For Topic B - 1MinAung HlaingNo ratings yet
- 4 Properties of Materials: Rs 12 Atomic and Molecular WeightsDocument4 pages4 Properties of Materials: Rs 12 Atomic and Molecular WeightsDilnesa EjiguNo ratings yet
- Oxidation States of The ElementsDocument8 pagesOxidation States of The ElementsYourMotherNo ratings yet
- Electronic Arrangement and ConfigurationDocument1 pageElectronic Arrangement and ConfigurationDrama QueenNo ratings yet
- 1 Q Ready Form PSPM 1 Sk015Document14 pages1 Q Ready Form PSPM 1 Sk015WAN NUR ALEEYA TASNIM BINTI WAN MOHAMED HAZMAN MoeNo ratings yet
- Ionic Radius - Wikipedia PDFDocument29 pagesIonic Radius - Wikipedia PDFடேவிட் ஸ்No ratings yet
- Configuración Electrónica de Los ElementosDocument5 pagesConfiguración Electrónica de Los ElementosArlette CedilloNo ratings yet
- Tabla de Configuración ElectrónicaDocument4 pagesTabla de Configuración ElectrónicaCesarPazoNo ratings yet
- Ques & Ans Pka KMLDocument21 pagesQues & Ans Pka KMLMuganeshNo ratings yet
- History and Subatomic Particle Review Take Two KEYDocument5 pagesHistory and Subatomic Particle Review Take Two KEYAlliya DaymonNo ratings yet
- Electron Config WSDocument3 pagesElectron Config WSCel blazNo ratings yet
- Ec of First 30 ElementsDocument6 pagesEc of First 30 ElementsJHANVI JADEJANo ratings yet
- Chembuddy AnswerDocument67 pagesChembuddy AnswerNATASHA 'ALIA BINTI ZULKIFLINo ratings yet
- Guyp SK015 22-23Document7 pagesGuyp SK015 22-23Farena LazimNo ratings yet
- Atomic MassDocument1 pageAtomic MassDeepti JainNo ratings yet
- MetallurgyDocument39 pagesMetallurgyPrabhakar BandaruNo ratings yet
- Oxidation State R RDocument13 pagesOxidation State R RManolo DavidNo ratings yet
- Unit Kimia Kolej Matrikulasi Kedah: SK 015, Chemistry Unit, KMK Pra PSPM Set 1Document7 pagesUnit Kimia Kolej Matrikulasi Kedah: SK 015, Chemistry Unit, KMK Pra PSPM Set 1aNo ratings yet
- 1 Cambridge IX Chem Unit 10.2 Redox ReactionsDocument32 pages1 Cambridge IX Chem Unit 10.2 Redox ReactionsSrihaan MathurNo ratings yet
- 9. Electrochemistry 1 ห้องฟ้าอิเล็ก หลังเรียนDocument28 pages9. Electrochemistry 1 ห้องฟ้าอิเล็ก หลังเรียนFelize IceNo ratings yet
- Relative Atomic Mass ConstantDocument2 pagesRelative Atomic Mass ConstantKhairul ZainuddinNo ratings yet
- Ores and Metallurgy-02 - Solved ProblemsDocument11 pagesOres and Metallurgy-02 - Solved ProblemsRaju SinghNo ratings yet
- Chemical CrosswordDocument3 pagesChemical CrosswordMary graceNo ratings yet
- Chemical Crossword No. 2 RulesDocument3 pagesChemical Crossword No. 2 RulesMary graceNo ratings yet
- Corrosion Basics: 2.1 Why Metals CorrodeDocument5 pagesCorrosion Basics: 2.1 Why Metals CorrodesadsadsadNo ratings yet
- Geochimie Curs 1Document5 pagesGeochimie Curs 1Istrate IsabelaNo ratings yet
- Structure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsFrom EverandStructure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsNo ratings yet
- Metal Powders: A Global Survey of Production, Applications and MarketsFrom EverandMetal Powders: A Global Survey of Production, Applications and MarketsNo ratings yet
- Analysis of the New Metals: Titanium, Zirconium, Hafnium, Niobium, Tantalum, Tungsten and Their AlloysFrom EverandAnalysis of the New Metals: Titanium, Zirconium, Hafnium, Niobium, Tantalum, Tungsten and Their AlloysNo ratings yet
- The Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyFrom EverandThe Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyNo ratings yet
- Advances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookFrom EverandAdvances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookArmando J. L. PombeiroRating: 5 out of 5 stars5/5 (1)
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Hydrogen in Steel: Effect of Hydrogen on Iron and Steel During Production, Fabrication, and UseFrom EverandHydrogen in Steel: Effect of Hydrogen on Iron and Steel During Production, Fabrication, and UseNo ratings yet
- Fossil Hydrocarbons: Chemistry and TechnologyFrom EverandFossil Hydrocarbons: Chemistry and TechnologyRating: 3 out of 5 stars3/5 (1)
- The Determination of Impurities in Nuclear Grade Sodium MetalFrom EverandThe Determination of Impurities in Nuclear Grade Sodium MetalNo ratings yet
- Values of Selected Physical Constants: Quantity Symbol SI Units Cgs UnitsDocument1 pageValues of Selected Physical Constants: Quantity Symbol SI Units Cgs UnitsSAUDNo ratings yet
- Course MME344Document1 pageCourse MME344SAUDNo ratings yet
- Mechanical Properties of Various Investment Casting Alloys 1-2Document1 pageMechanical Properties of Various Investment Casting Alloys 1-2SAUDNo ratings yet
- Characteristics of Selected ElementsDocument1 pageCharacteristics of Selected ElementsSAUDNo ratings yet
- Kitz PDFDocument20 pagesKitz PDFAde MustiaharjaNo ratings yet
- A Comparative Study of Spectral Data and Color Fastness On 100% Linen and 100% Cotton Dyed With Reactive Dye in CPB AND PDPS Dyeing ProcessDocument62 pagesA Comparative Study of Spectral Data and Color Fastness On 100% Linen and 100% Cotton Dyed With Reactive Dye in CPB AND PDPS Dyeing ProcessDesign ZoneNo ratings yet
- MAS Sakti Email:: 600WOG/150WSP Full Port Two-Piece Brass Ball ValvesDocument4 pagesMAS Sakti Email:: 600WOG/150WSP Full Port Two-Piece Brass Ball ValvesAli SyahbanaNo ratings yet
- We Know How: Carbon SteelDocument4 pagesWe Know How: Carbon SteelMohamed RaafatNo ratings yet
- WeldedBridgeCode ACS 2 6Document1 pageWeldedBridgeCode ACS 2 6MAYMODERN STEELNo ratings yet
- (R D Shannon) Chemical Bonding in Solids (B-Ok - CC) PDFDocument171 pages(R D Shannon) Chemical Bonding in Solids (B-Ok - CC) PDFJaga ParamunitaNo ratings yet
- Kut Thioseal 227: Two Component Gun and Pouring Grade Polysulfide SealantDocument4 pagesKut Thioseal 227: Two Component Gun and Pouring Grade Polysulfide Sealanthafee83No ratings yet
- 1613 Nova NiroDocument1 page1613 Nova NiroAhmedRamadanNo ratings yet
- Casting Fabrication of Carbon Steel Pitman For Crusher Suppliers and Manufacturers China - Professional Factory - Zhengda HDocument1 pageCasting Fabrication of Carbon Steel Pitman For Crusher Suppliers and Manufacturers China - Professional Factory - Zhengda HCarlos Ediver Arias RestrepoNo ratings yet
- TL 211 Exterior and Plastics PaintDocument8 pagesTL 211 Exterior and Plastics PaintTríHoàngNo ratings yet
- Api 653 Tank Inspection, Tank Maintenance, AND Causes of Tank FailureDocument43 pagesApi 653 Tank Inspection, Tank Maintenance, AND Causes of Tank FailureArif PriyadiNo ratings yet
- Hum1 PrelimDocument78 pagesHum1 PrelimJohara BayabaoNo ratings yet
- Piping Basis of DesignDocument19 pagesPiping Basis of DesignMajid DixonNo ratings yet
- Soal B.inggris UjianDocument14 pagesSoal B.inggris UjianFastabikul KhairatNo ratings yet
- Pamphlet (DW 2594)Document1 pagePamphlet (DW 2594)Irvansyah RazadinNo ratings yet
- A.02 Layout Rev 1Document1 pageA.02 Layout Rev 1Louis KiwaNo ratings yet
- BOQ of Construction of GateDocument8 pagesBOQ of Construction of Gatemichael jan de celisNo ratings yet
- Microstructure, Mechanical Properties and Wear Behavior of Metallic, Nonmetallic and Deep Cryogenically Chilled ASTM A216 WCB SteelDocument8 pagesMicrostructure, Mechanical Properties and Wear Behavior of Metallic, Nonmetallic and Deep Cryogenically Chilled ASTM A216 WCB SteelVeluswamy VeerappanNo ratings yet
- Materials Varieties and ApplicationsDocument2 pagesMaterials Varieties and ApplicationsHossein GhazinezhadNo ratings yet
- Alpolic FRDocument48 pagesAlpolic FRM. Murat ErginNo ratings yet
- Technical Manual LU 8047 TMDocument6 pagesTechnical Manual LU 8047 TMArif ZuhairiNo ratings yet
- Managing An On-Premises LaundryDocument4 pagesManaging An On-Premises LaundryGil TeodosipNo ratings yet
- Ultracote 635 HBDocument3 pagesUltracote 635 HBCherbee Ferrer100% (1)
- Bulk Deformation ProcessesDocument41 pagesBulk Deformation ProcessesAbdullahNo ratings yet
- Day3 1 PDFDocument15 pagesDay3 1 PDFBishwajyoti Dutta MajumdarNo ratings yet
- Carding Setting Between LickerDocument3 pagesCarding Setting Between LickerRAHEEL JAVEDNo ratings yet
- Properties of Period 3 Elements and Their Oxides QPDocument11 pagesProperties of Period 3 Elements and Their Oxides QPfatma sNo ratings yet
- Fossil Fuels: Grade Level: 4 - 6Document12 pagesFossil Fuels: Grade Level: 4 - 6api-438357435No ratings yet
- Quality 11Smnpb37: Lucefin GroupDocument1 pageQuality 11Smnpb37: Lucefin GroupPaulo ZechinNo ratings yet