0% found this document useful (0 votes)
2K views7 pagesPeriodic Table - Problem Solving - JEE Sheet PDF
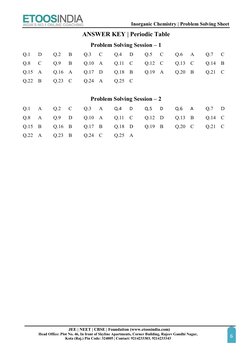
This document provides 20 multiple choice questions regarding periodic trends and properties related to the periodic table. Specifically, it covers topics like electronic configurations, atomic and ionic radii trends, ionization energies, effective nuclear charge, and screening effects. The questions are intended to help students practice applying their knowledge of the periodic table to solve problems involving periodic trends and properties.
Uploaded by
edCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
2K views7 pagesPeriodic Table - Problem Solving - JEE Sheet PDF
This document provides 20 multiple choice questions regarding periodic trends and properties related to the periodic table. Specifically, it covers topics like electronic configurations, atomic and ionic radii trends, ionization energies, effective nuclear charge, and screening effects. The questions are intended to help students practice applying their knowledge of the periodic table to solve problems involving periodic trends and properties.
Uploaded by
edCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
- Problem Solving Session 1
- Problem Solving Session 2
- Answer Key