Professional Documents
Culture Documents
Ure Substance: Saturation Temperature (T)
Uploaded by
Muhammad MasroorOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ure Substance: Saturation Temperature (T)
Uploaded by
Muhammad MasroorCopyright:
Available Formats
Chapter#2: Properties of pure substances
PURE SUBSTANCE
1. A substance that has a fixed chemical composition throughout is called pure substance.
2. Example:
a. Liquid water
b. Liquid water and ice
c. Liquid water, and steam
d. Gases (N2, CO2)
e. Air
3. Following are not the pure substances:
a. Oil + water
b. Air unmixed with water
c. Steam with impurities
PHASES
(a) Solid (b) Liquid (c) Gas
PHASES OF WATER
a) Compressed liquid or subcooled liquid (not about to
vaporize)
b) Saturated liquid (about to vaporize)
c) Saturated or vapor-liquid mixture (liquid and vapor
phases co-exist)
d) Saturated vapors (about to condense)
e) Superheated vapors (not about to condense)
PROBLEM
What is the difference between gas, vapors and steam?
SATURATION TEMPERATURE AND PRESSURE
Saturation temperature (Tsat)
1. It is the temperature at which a pure substance changes its phase.
2. Example:
a. Water boils at 100°C (Tsat) at P = 1 atm
b. Water boils at 250°C (Tsat) at P = 4 atm
Saturation pressure (Psat)
3. It is the pressure at which a pure substance changes its phase.
4. Example:
a. Water boils at 1 atm (Psat) at T = 100°C
b. Water boils at 0.0068 atm (Psat) at T = 30°C
Handouts for Thermodynamics 2
Chapter#2: Properties of pure substances
LATENT HEATS
The amount of heat absorbed or released during phase-change process is called latent heat.
Latent heat of fusion
It is the heat which is absorbed during melting or released during freezing of a pure substance.
E.g. latent heat of fusion for water is (approx..) 333 kJ/kg at 1 atm.
ICE LIQUID
Latent heat of vaporization (hfg)
It is the heat which is absorbed during vaporization or released during condensation of a pure substance.
E.g. latent heat of vaporization for water is (approx..) 2257 kJ/kg at 1 atm.
LIQUID VAPORS
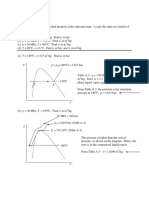
PROPERTY DIAGRAM FOR THE PHASE-CHANGE PROCESS (WATER)
1. Higher is the pressure (P):
a. higher is saturation temperature (Tsat)
b. more is specific volume of saturation liquid
c. less is specific volume of saturation vapors
2. At critical point where Pcr=22.09 MPa and Tcr=375.14°C, saturation liquid and saturation vapors
states are identical i.e. vcr = 0.003155 m3/kg.
Handouts for Thermodynamics 3
Chapter#2: Properties of pure substances
T-v and P-v property diagrams
Handouts for Thermodynamics 4
Chapter#2: Properties of pure substances
P-V DIAGRAM EXTENDING TO INCLUDE SOLID PHASE
For substances that contracts on cooling
For substances that expand on cooling (e.g. water)
1. On a triple line, all three phases co-exist
2. If the pressure is lower than triple line, sublimation will occur.
Handouts for Thermodynamics 5
Chapter#2: Properties of pure substances
P-T diagram
1. TTP = Triple point temperature PTP = Triple point pressure
2. TTP and PTP are inherent property of a pure substance
3. For water: TTP = 0.01°C and PTP = 0.6113kPa
4. Special case of dry-ice (CO2): It has triple point pressure (517 kPa) higher than atmospheric pressure
(101 kPa) so it sublimates (i.e. change its phase from solid to vapors directly, without getting
liquefied).
PROBLEMS
1. A pure substance has triple point pressure 0.3bar. It is placed in a chamber where the pressure is
0.01bar and a temperature is such that it is in solid phase. On heating, it first melts and then vaporizes.
Is it possible?
2. A pure substance melts at T=400°C and then vaporize at 340°C at a pressure higher than triple point
pressure. Is it possible?
Answer to both the problems is No.
ENTHALPY
1. The total heat content of a system is called enthalpy.
2. It is a combination property.
H = U + PV [kJ]
h = u + Pv [kJ/kg]
Handouts for Thermodynamics 6
Chapter#2: Properties of pure substances
PROPERTY TABLES
Subscripts: f = fluid or liquid g = gas or vapors fg = g – f
A-4: For saturated liquid and saturated vapor states when T is given
A-5: For saturated liquid and saturated vapor states when P is given
A-6: For superheated water
A-7: For subcooled water (optional)
Linear interpolation
Y = (Y2 – Y1)/(X2 – X1) x (X-X1) + Y1
SATURATED LIQUID AND SATURATED VAPOR STATES
Use property tables A-4 and A-5
PROBLEM
A rigid tank contains 50 kg of saturated liquid water at 90°C. Determine pressure in the tank and volume
of tank.
Solution:
m = 50 kg T = 90°C State: Saturated liquid
P = Psat@T=90°C = ? V=?
P = Psat@T=90°C = 70.14 kPa
V = m.v = m . vf@T=90°C = 50 x 0.001036 = 0.0518 m3
PROBLEM
A mass of 200 g of saturated liquid water is completely vaporized at a constant pressure of 100 kPa.
Determine the volume change and the amount of energy added to water.
Solution:
m = 0.2 kg P = 100 kPa
States: (1) = Saturated liquid (2) = Saturated vapors
∆V = ? ∆H = ?
∆V = V2 – V1 = m(v2-v1) = m(vg - vf) = m.vfg = m.vfg@P=100kPa = 0.2 x 1.6930
= 0.3386 m3
∆H = H2 – H1 = m(h2-h1) = m(hg - hf) = m(hg@P=100kPa – hf@P=100kPa )= 451.6 kJ
Note: hfg is called enthalpy of vaporization or latent heat of vaporization.
Handouts for Thermodynamics 7
Chapter#2: Properties of pure substances
SATURATED LIQUID-VAPOR MIXTURE (VLM)
Use property tables A-4, A-5 and quality.
Quality
1. It defines the mass of vapors present in a VLM state.
2. x = mvapor / mtotal = mvap / ( mvap + mliq) = mg / (mg + mf) = mg / mt
3. The value of x is in between 0 and 1.
x = 0 means saturation liquid x = 1 means saturation vapors
Average properties
The liquid and vapors in VLM states are mixed so well that they form a
homogeneous mixture. Therefore, their properties can be taken as
average.
Such that yav = yf + x.yfg and Yav = mt.yav
Also Yf = mf.yf Yg = mg.yg Yav = Yf + Yg
Where yf yav yg
Examples: vav = vf + x.vfg hav = hf + x.hfg uav = uf + x.ufg
PROBLEM
What is specific volume of water at 100°C and 30% quality?
Hint:
Find vf and vg at 100°C from Table A-4 and then calculate vav at x = 0.3.
PROBLEM
At some constant temperature, if vf = 0.002 m3/kg, vg = 0.2 m3/kg and vav = 0.11 m3/kg, calculate x and
plot state on T-v diagram.
Solution:
x = (0.11 – 0.002) / (0.2 – 0.002) = 54%
PROBLEM
A rigid tank contains 10 kg of water at 90°C. If 8 kg of water is in liquid form and the rest is in vapor
form, determine pressure in the tank and volume of tank.
Solution:
mt = 10 kg mf = 2 kg
mg = 8 kg and x = mg / mt = 0.2
T = 90°C State: VLM
P=? V=?
Handouts for Thermodynamics 8
Chapter#2: Properties of pure substances
P = Psat@T=90°C = 70.14 kPa
V = mt . vav
where, vav = vav@x=0.2 = vf@T=90°C + x . vfg@T=90°C = 0.001036 + 0.2 x (2.359964) = 0.473 m3/kg
Hence, V = 10 x 0.473 = 4.73 m3
SUPERHEATED WATER
Use property table A-6.
Given problem must be satisfying the following conditions:
T > Tsat (at given P)
P < Psat (at given T)
v > vg, h > hg, u > ug (at given Tsat and Psat)
PROBLEMS
1. v = ? at T = 100°C and P = 0.05 MPa (Ans: 3.4187 m3/kg)
2. v = ? at T = 900°C and P = 7 MPa (Ans: 0.076750 m3/kg)
3. h = ? at T = 350°C and P = 0.01 MPa (Ans: 3178.35 kJ/kg)
P = 0.01 MPa (common)
T (known) h (required)
300 3076.7
350 (3076.7 + 3280)/2
→in mid = 3178.35
400 3280
4. u = ? at T = 250°C and P = 0.35 MPa
5. v = ? at T = 450°C and P = 0.7 MPa
Handouts for Thermodynamics 9
Chapter#2: Properties of pure substances
6. h = ? at T = 850°C and P = 9.5 MPa
7. v = ? at T = 110°C and P = 0.01 MPa
8. h = ? at T = 430°C and P = 0.7 MPa
9. P = ? at v = 10.828 m3/kg and T = 900°C
10. T = ? at P = 0.7 MPa and h = 3059.45 kJ/kg
11. T = ? at P = 11 MPa and u = 2920 kJ/kg
Handouts for Thermodynamics 10
Chapter#2: Properties of pure substances
PROBLEM
What is the phase and temperature of water at 0.5 MPa and 2890 kJ/kg enthalpy.
Solution:
As state is unclear therefore, a check is required.
h = h@p=0.5MPa = 2890 kJ/kg
hg = hg@P=0.5MPa = 2784 kJ/kg
As h > hg therefore, state is superheated vapors.
(Solve further)
COMPRESSED LIQUID WATER
Use property table A-7.
Given problem must be satisfying the following conditions:
T < Tsat (at given P)
P > Psat (at given T)
v < vf, h < hf, u < uf (at given Tsat and Psat)
Approximation: y = yf@T (use table A-1)
PROBLEM
P = 8 MPa T = 65°C h=?
(Answer: 278.68 kJ/kg)
PROBLEM
Determine the internal energy of compressed liquid water at 80°C and 5 MPa using (a) data from
compressed liquid tables (b) data from saturated liquid tables. Also calculate error involved.
Solution:
(a) u = u@T=80°C,P=5MPa = 333.82 kJ/kg
(b) u = uf@T=80°C = 334.97 kJ/kg
Error = (334.97 - 333.82 ) / 333.82 x 100 = 0.34% < 1% !!!
Handouts for Thermodynamics 11
Chapter#2: Properties of pure substances
CONSTANT SPECIFIC VOLUME HEATING
Hint: P = const. or T = const. is not mentioned.
PROBLEM
A rigid tank with a volume of 2.5m3 contains 5 kg of saturated liquid-vapor mixture of water at 75°C.
Now, the water is slowly heated. Determine the temperature at which the liquid in the tank is completely
vaporized. Also, show the process on a T-v diagram.
Solution:
V = 25 m3 m = 5 kg => v = 2.5/5 = 0.5 m3/kg
T = Tsat@vg=v=0.5 = ?
(Answer: 140.7 °C)
IDEAL GAS EQUATION OF STATE
Equation of state
1. Any equation which relates pressure, temperature and specific volume of a substance is called
equation of state.
2. Example:
a. Ideal gas equation of state
b. Van der Waals equation of state
c. Beattie-Bridgeman equation of state
d. Benedict-Webb-Rubin (BWR) equation of state
e. Viral equation of state
Ideal gas equation of state
Pv = RT
Where,
P = Absolute pressure [Pa]
T = Absolute temperature [K]
R = Gas constant = Ru / M [kJ/kgK]
Ru = Universal gas constant = 8.314 kJ/kmolK
M = Molar mass (e.g. N2 = 28 kg/kmol)
Note: See Table A-1 for values of R and M. Be careful about unit consistency.
Ideal gas equation between two states
P1 V1 / T1 = P2 V2 / T2
Ideal gas
1. It is an imaginary substance
Handouts for Thermodynamics 12
Chapter#2: Properties of pure substances
2. Real gases at low densities (Low pressure and high temperature) behaves close to ideal gas.
3. Water vapor at pressure less than 10 kPa, regardless of any temperature can be treated as ideal gas.
PROBLEM
Determine the mass of air in a room having dimensions 4m x 5m x 6m at 100 kPa and 25°C. Treat air as
an ideal gas with R=0.287 kPa.m3/kgK
Pv = RT => PV = mRT => m = PV/RT = … = 140.3 kg
SPECIFIC HEATS
Specific heat
1. It is the amount of specific energy required to raise the temperature of a substance by 1°C.
2. Unit: kJ/kgK or kJ/kg°C
3. It is a strong function of material and temperature.
Specific heat at constant pressure (Cp)
1. If the process is executed at constant pressure then, the amount of specific energy required to raise the
temperature of a substance by 1°C is called specific heat at constant pressure.
2. For example in open systems
3. Denoted by Cp = (∂h/∂T)p
Derivation:
ein – eout = esys
ein –eout = du + P.dv
CpdT = dh
Cp = (dh/dT)p
Also, h2 – h1 = h = Cp(T) dT
Specific heat at constant volume (Cv)
1. If the process is executed at constant volume then, the amount of specific energy required to raise the
temperature of a substance by 1°C is called specific heat at constant volume.
2. For example in close systems
3. Denoted by Cv = (∂u/∂T)v
Derivation:
ein – eout = esys
ein –eout = du
CvdT = du
Cv = (du/dT)v
Also, u2 – u1 = u = Cv(T) dT
Handouts for Thermodynamics 13
Chapter#2: Properties of pure substances
Specific heats relation
We know,
h = u + Pv
h = u + RT
dh = du+ RdT
Cp dT = Cv dT + R dT
Cp = Cv + R
Hence,
Cp > Cv
Specific heat ratio
k = Cp / Cv
REMEMBER
For air: Cp = 1.005 kJ/kgK R = 0.287 kJ/kgK Cv = 0.718 kJ/kgK k = 1.4
Handouts for Thermodynamics 14
You might also like
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- W-4, Chap.3-Properties of Pure Substances-2Document31 pagesW-4, Chap.3-Properties of Pure Substances-2سيمو بشيريNo ratings yet
- Revision Exercises 2 - AnswersDocument2 pagesRevision Exercises 2 - AnswersSal Sabeela RahmanNo ratings yet
- Steam Tank Pressure & QualityDocument5 pagesSteam Tank Pressure & QualityAri Reza KNo ratings yet
- Chapter 3 PDFDocument5 pagesChapter 3 PDFLily Antonette AgustinNo ratings yet
- Me 211 Examples SolutionsDocument30 pagesMe 211 Examples SolutionsBryan Dominic Gabriel PaduaNo ratings yet
- Property Tables + Equation of StateDocument66 pagesProperty Tables + Equation of StateTetiana VitenkoNo ratings yet
- The Working Fluid in ThermodynamicsDocument13 pagesThe Working Fluid in ThermodynamicsFarouk BassaNo ratings yet
- Thermodynamics - 1 Midterm SolutionDocument10 pagesThermodynamics - 1 Midterm SolutionEarl Maxie Lagdamin ErederaNo ratings yet
- PROPERTY TABLES + EQUATION OF STATE GUIDEDocument66 pagesPROPERTY TABLES + EQUATION OF STATE GUIDEMuhammad Randy AkbarNo ratings yet
- Thermodynamics: Practice Problems For Property EvaluationDocument9 pagesThermodynamics: Practice Problems For Property EvaluationSaurav kumar silNo ratings yet
- Borgnakke's Fundamentals of Thermodynamics: Global EditionDocument67 pagesBorgnakke's Fundamentals of Thermodynamics: Global Edition정윤서No ratings yet
- Moist Air Properties and Air Conditioning ProcessesDocument37 pagesMoist Air Properties and Air Conditioning ProcessesAlex ChanNo ratings yet
- Chapter 1 - ContentDocument85 pagesChapter 1 - ContentMalik KirbyNo ratings yet
- Thermodynamics 1 - Properties of Pure SubstancesDocument26 pagesThermodynamics 1 - Properties of Pure SubstancesFlorasaurus1767% (3)
- Reviewlecture-I 20081001 48e3c2399f4d65 74115154Document37 pagesReviewlecture-I 20081001 48e3c2399f4d65 74115154Austin BarrilleauxNo ratings yet
- Chemical Engineering Principles SATsDocument7 pagesChemical Engineering Principles SATsAli Hamza ManzoorNo ratings yet
- Properties of Pure Substances GuideDocument9 pagesProperties of Pure Substances GuideMahmoud AbdelghfarNo ratings yet
- Thermo NotesDocument16 pagesThermo NotesjecuadranteNo ratings yet
- hw#3 SolutionsDocument7 pageshw#3 SolutionsZumaflyNo ratings yet
- 10 4 AnnotatedDocument48 pages10 4 AnnotatedkwandoossNo ratings yet
- Module 3 (Lesson 1 and 2)Document10 pagesModule 3 (Lesson 1 and 2)rocky.mercado2No ratings yet
- Topic1-1 Thermal PrincipleDocument40 pagesTopic1-1 Thermal PrincipleEdith Carumbana JusayanNo ratings yet
- HW3 SolutionDocument14 pagesHW3 SolutionBryceNo ratings yet
- Properties of Pure SubstanceDocument32 pagesProperties of Pure SubstanceMaherNo ratings yet
- 2 - Properties of Pure SubstanceDocument39 pages2 - Properties of Pure Substancerashedramadan46No ratings yet
- Science ReviewerDocument3 pagesScience ReviewerPamee BautistaNo ratings yet
- PHASEDocument57 pagesPHASEKarl SiaganNo ratings yet
- Presentation ReportDocument8 pagesPresentation ReportJackson ChongNo ratings yet
- EME 2315 Chapter 2Document10 pagesEME 2315 Chapter 2Andy OchiengNo ratings yet
- Gas Laws ExplainedDocument18 pagesGas Laws ExplainedMinn SunnNo ratings yet
- Thermodynamics 1 by Sta. Maria Chapter 3 Solution ManualDocument7 pagesThermodynamics 1 by Sta. Maria Chapter 3 Solution ManualAllen MalabarbasNo ratings yet
- 2 - ProcessesDocument36 pages2 - ProcessesAljohn Mark ReyesNo ratings yet
- M2A1 Thermodynamic Properties (Assignment) JSMDocument4 pagesM2A1 Thermodynamic Properties (Assignment) JSMUmair AbbasNo ratings yet
- The T-DS Equations & DiagramsDocument58 pagesThe T-DS Equations & DiagramsWoo GongNo ratings yet
- Handout 9 ThermodynamicsDocument10 pagesHandout 9 ThermodynamicsMary Grace AcostaNo ratings yet
- Engg ThermodynamicsgfDocument3 pagesEngg Thermodynamicsgfphysics a2No ratings yet
- Ideal Gas Laws and Thermodynamic PropertiesDocument6 pagesIdeal Gas Laws and Thermodynamic PropertiesJoshua EspirituNo ratings yet
- W-3, Chap.3-Properties of Pure Substances-1Document33 pagesW-3, Chap.3-Properties of Pure Substances-1سيمو بشيريNo ratings yet
- Thermodynamic 2019-2: Nedher Sánchez RamírezDocument47 pagesThermodynamic 2019-2: Nedher Sánchez RamírezGenesis FloresNo ratings yet
- Chapter 4 Heat ReviewDocument16 pagesChapter 4 Heat ReviewKurdishNo ratings yet
- Basic Concept ThermodynamicsDocument62 pagesBasic Concept Thermodynamicscjdbbt1No ratings yet
- Thermodynamic Units & Properties of WaterDocument7 pagesThermodynamic Units & Properties of WaterRekha ToshniwalNo ratings yet
- T P Const. V Const: F FG F FGDocument4 pagesT P Const. V Const: F FG F FGAiena AzlanNo ratings yet
- FE Thermodynamics Review 2011Document31 pagesFE Thermodynamics Review 2011Engr Amethyst RiegoNo ratings yet
- ES 31 - Thermodynamics and Heat TransferDocument5 pagesES 31 - Thermodynamics and Heat Transferyeng botzNo ratings yet
- Thermochemistry (Solutions)Document16 pagesThermochemistry (Solutions)MarikNo ratings yet
- q m C ΔT: SolutionDocument7 pagesq m C ΔT: SolutionMjhay Tanchiatco DavidNo ratings yet
- ChapterII - GasesDocument40 pagesChapterII - Gasesjumanahelmy12No ratings yet
- Basic State Values of Matter: Example 1.1Document27 pagesBasic State Values of Matter: Example 1.1Warren CabunyagNo ratings yet
- Thermodynamics: Lecture 2 Review of Lecture 1Document10 pagesThermodynamics: Lecture 2 Review of Lecture 1Lexter Gomez GabicaNo ratings yet
- C 3Document14 pagesC 3jfl2096No ratings yet
- ch03 JmsDocument39 pagesch03 Jmsdr. waleed ElbehairyNo ratings yet
- Thermodynamics 1 LEC-3Document17 pagesThermodynamics 1 LEC-3Ariharan KumaranNo ratings yet
- Thermodynamics: Conservation of EnergyDocument38 pagesThermodynamics: Conservation of EnergyAron H OcampoNo ratings yet
- Fluid Mechanics Cengel (Solutions Manual) Chap12-001Document34 pagesFluid Mechanics Cengel (Solutions Manual) Chap12-001NURUL SYUHADA BT ISMAIL HAJAR50% (2)
- Sample Problems - Pure SubstanceDocument6 pagesSample Problems - Pure SubstanceRonalie DavaNo ratings yet
- Phase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringFrom EverandPhase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringNo ratings yet
- User Manual English: Ground TestersDocument44 pagesUser Manual English: Ground TestersbereniceNo ratings yet
- Aerslim 35 HD - TehnikaDocument4 pagesAerslim 35 HD - Tehnikaikac3No ratings yet
- A6d800 Ae05 03 EngDocument6 pagesA6d800 Ae05 03 EngAdemar FukeNo ratings yet
- EXP1-SOUND INTENSITY MEASUREMENT-non GuidedDocument4 pagesEXP1-SOUND INTENSITY MEASUREMENT-non GuidedRyan LauNo ratings yet
- Glossary CTDocument7 pagesGlossary CTnikkoelbao96No ratings yet
- Flow Mobile CalibartionDocument2 pagesFlow Mobile CalibartionMohit Jadon100% (1)
- Building 261Document2 pagesBuilding 261OvyeNo ratings yet
- Question Bank BEEDocument5 pagesQuestion Bank BEEK.Sushita VISTASNo ratings yet
- OVIA SensorDocument1 pageOVIA Sensortasqatar contractingNo ratings yet
- Mi Unit IiDocument131 pagesMi Unit IiNithish kumar RajendranNo ratings yet
- What Is The Pressure at The End of A Pipe Which Is Discharging To AtmosphereDocument2 pagesWhat Is The Pressure at The End of A Pipe Which Is Discharging To AtmospheremwardNo ratings yet
- The Special Theory of Relativity: Class Notes On Applied Modern Physics - ECEG 2101Document16 pagesThe Special Theory of Relativity: Class Notes On Applied Modern Physics - ECEG 2101Gébrè Sîllãsíê100% (1)
- Ebook-Learn Thermal Analysis With Altair OptiStructDocument114 pagesEbook-Learn Thermal Analysis With Altair OptiStructRAHUL K SEC 2020No ratings yet
- Wa0002.Document18 pagesWa0002.Sakthi MadNo ratings yet
- EME 2315 Chapter 5Document9 pagesEME 2315 Chapter 5Andy OchiengNo ratings yet
- Topics: Ages (Eqm) : Bank MathDocument14 pagesTopics: Ages (Eqm) : Bank Mathbiltum66472No ratings yet
- Topic 3 Test - Paper 2Document2 pagesTopic 3 Test - Paper 2IB BaddiesNo ratings yet
- UNITS OF MEASUREMENT MCQSDocument6 pagesUNITS OF MEASUREMENT MCQSAzamNo ratings yet
- Erlang B TablDocument3 pagesErlang B TablaehwnnnNo ratings yet
- 9mm!ূຌዡገ๕ۉ࿋ഗ Rotary Potentiometers With Metal Shaft Series: WH9011A-1-18TDocument26 pages9mm!ূຌዡገ๕ۉ࿋ഗ Rotary Potentiometers With Metal Shaft Series: WH9011A-1-18TpeterfunNo ratings yet
- Lecture Notes 1 - Fluid Mechanics - 1Document7 pagesLecture Notes 1 - Fluid Mechanics - 1Jane AndaNo ratings yet
- PT PLN Battery Monitoring ReportDocument7 pagesPT PLN Battery Monitoring Reportmuhammad lendiNo ratings yet
- Termodinámica Química Aplicada problemasDocument16 pagesTermodinámica Química Aplicada problemasakksNo ratings yet
- Exam PaperDocument1 pageExam PapernoseyuniverseNo ratings yet
- JEE Main 2021 Question Paper Physics Feb 24 Shift 2Document16 pagesJEE Main 2021 Question Paper Physics Feb 24 Shift 2B Srinivas.No ratings yet
- Physics ReviewerDocument21 pagesPhysics ReviewerainnahNo ratings yet
- 3 Introduction To EEDocument2 pages3 Introduction To EEganesanNo ratings yet
- Public PM5350 v1.11.0 Register ListDocument31 pagesPublic PM5350 v1.11.0 Register ListTam DuongNo ratings yet
- Home Assignment - Experimental Methods & Analysis: Ia Random Variables and Probability DistributionsDocument23 pagesHome Assignment - Experimental Methods & Analysis: Ia Random Variables and Probability DistributionstotiNo ratings yet
- UGNA3023 Applied Hydraulics Practical 1: Investigation of Flow and Pressure Drop in A Pipe and Across FixturesDocument6 pagesUGNA3023 Applied Hydraulics Practical 1: Investigation of Flow and Pressure Drop in A Pipe and Across Fixtures木辛耳总No ratings yet