Professional Documents
Culture Documents
Chylothorax Treatment Planning
Uploaded by
Vianney Olvera0 ratings0% found this document useful (0 votes)
15 views10 pagesCopyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
15 views10 pagesChylothorax Treatment Planning
Uploaded by
Vianney OlveraCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 10
New 2016
American College of Radiology
ACR Appropriateness Criteria®
Chylothorax Treatment Planning
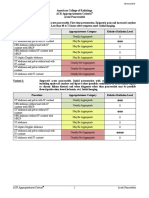
Variant 1: Chylothorax treatment planning: traumatic etiology.
Radiologic Procedure Rating Comments RRL*
This procedure is the initial examination
X-ray chest 8 to screen for pleural effusion or an ☢
alternative cause of dyspnea or chest pain.
If further evaluation and minimally
invasive treatment is warranted, this
Lymphangiography chest and abdomen 8 procedure is the test of choice for ☢☢☢
traumatic chylothorax and can include
diagnostic and therapeutic embolization.
This procedure is particularly helpful if
MRI chest and abdomen without IV
6 lymphangiography does not delineate an O
contrast
abnormality.
This procedure is particularly helpful if
MRI chest and abdomen without and with
5 lymphangiography does not delineate an O
IV contrast
abnormality.
CT chest and abdomen without IV
5 ☢☢☢☢
contrast
CT chest and abdomen without and with
5 ☢☢☢☢
IV contrast
CT chest and abdomen with IV contrast 4 ☢☢☢☢
Lymphoscintigraphy chest and abdomen 4 ☢☢
US chest and abdomen 4 O
*Relative
Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate
Radiation Level
ACR Appropriateness Criteria® 1 Chylothorax Treatment Planning
Variant 2: Chylothorax treatment planning: nontraumatic or unknown etiology.
Radiologic Procedure Rating Comments RRL*
This procedure is the initial examination
X-ray chest 8 to screen for pleural effusion or an ☢
alternative cause of dyspnea or chest pain.
This procedure is appropriate if a
Lymphangiography chest and abdomen 8 concomitant minimally invasive attempt at ☢☢☢
therapy is desired.
MRI chest and abdomen without IV This procedure is useful to visualize
7 O
contrast lymphatic vessels.
This procedure is useful to visualize
MRI chest and abdomen without and with
7 lymphatic vessels and exclude vascular O
IV contrast
abnormalities.
This procedure is helpful if venous
CT chest and abdomen with IV contrast 7 thrombosis is suspected as the cause of the ☢☢☢☢
chylous effusion.
CT chest and abdomen without IV
5 ☢☢☢☢
contrast
CT chest and abdomen without and with
5 ☢☢☢☢
IV contrast
Lymphoscintigraphy chest and abdomen 3 ☢☢
US chest and abdomen 3 O
*Relative
Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate
Radiation Level
ACR Appropriateness Criteria® 2 Chylothorax Treatment Planning
CHYLOTHORAX TREATMENT PLANNING
Expert Panel on Vascular Imaging and Interventional Radiology: Bill S. Majdalany, MD 1;
Douglas A. Murrey Jr, MD, MS2; Baljendra S. Kapoor, MD3; Thomas R. Cain, MD4; Suvranu Ganguli, MD5;
Michael S. Kent, MD6; Fabien Maldonado, MD7; Joseph J. McBride, MD8; Jeet Minocha, MD9;
Stephen P. Reis, MD10; Jonathan M. Lorenz, MD11; Sanjeeva P. Kalva, MD.12
Summary of Literature Review
Introduction/Background
Chyle is primarily formed in the intestines and is composed of proteins, lipids, electrolytes, and lymphocytes. A
chylous pleural effusion, or chylothorax, is a highly morbid condition defined by the presence of chyle within the
pleural space. Chronic chyle leak results in metabolic abnormalities, respiratory compromise,
immunosuppression, malnutrition, and even death [1-3]. A review of the etiology, diagnosis, and management of
chylothorax is presented in addition to an evaluation of relevant imaging studies.
Etiology
Chylothoraces can be categorized etiologically as traumatic or nontraumatic. Collectively, the incidence of
chylothorax is approximately 1 per 6000 admissions [1]. Historically, nontraumatic etiologies accounted for up to
72% of cases. Most recently, the largest study reports that traumatic etiologies account for 54% of cases [1,4-7].
The discrepancy may reflect the growth in thoracic oncologic resections or specific referral patterns.
Diagnosis
Chylothorax most commonly presents with dyspnea, although chest pain, fever, and fatigue may also occur. Chyle
is odorless, alkaline, sterile, and milky in appearance, although the appearance may vary based on the nutritional
status of the patient. Increasing fatty intake increases the volume and can change the color of the fluid and has
been described for the diagnosis of a chyle leak. The hallmark of chylous effusion is the presence of chylomicrons
in the fluid. Objective diagnostic criteria include a pleural fluid triglyceride level >110 mg/dL and a ratio of
pleural fluid to serum triglyceride level of >1.0. A ratio of pleural fluid to serum cholesterol level of <1.0
distinguishes chylothorax from cholesterol pleural effusions, which may present similarly [2,3].
Management
The diagnosis is confirmed by draining the fluid for studies; this is also palliative. After replacing fluid and
protein losses, a decision about conservative versus invasive therapies can be made. If the chylothorax
reaccumulates, treatment is guided by daily outputs, with higher outputs warranting a more aggressive approach
[2,4,8-11].
Conservative measures include management of the underlying disease, thoracentesis, and dietary modifications
such as total parenteral nutrition or a nonfat diet to reduce production of chyle and consequently flow through the
thoracic duct. Adjunctive therapy may include somatostatin, etilefrine, or nitric oxide, with the underlying
etiology determining the efficacy, although the evidence remains scarce. The success of conservative therapy
approaches 50% in nonmalignant etiologies but is only minimally beneficial in neoplastic etiologies [2,10,11].
Exact criteria for the implementation of invasive treatment are not well defined, but several authors advocate its
use if conservative treatment has not resolved the chylothorax after 2 weeks, in higher-output chylothoraces, and
in underlying neoplastic etiologies. Invasive treatments include surgical thoracic duct ligation, pleurodesis, and
thoracic duct embolization (TDE) [2,4,8-11]. Less commonly, tunneled drains or pleural shunt procedures are
performed, although prolonged drainage is not recommended as a long-term option because of increased risk of
complications [12,13]. Although the technical success of direct surgical ligation is high, these debilitated patients
1
Principal Author and Panel Vice-Chair (Vascular Imaging), University of Michigan Health System, Ann Arbor, Michigan. 2Research Author, University of
Michigan, Ann Arbor, Michigan. 3Panel Chair (Interventional Radiology), Cleveland Clinic Foundation, Cleveland, Ohio. 4Yuma Regional Medical Center,
Yuma, Arizona. 5Massachusetts General Hospital, Boston, Massachusetts. 6Beth Israel Deaconess Medical Center, Boston, Massachusetts, Society of
Thoracic Surgeons. 7Vanderbilt University Medical Center, Nashville, Tennessee, American College of Chest Physicians. 8University of Nebraska Medical
Center, Omaha, Nebraska. 9University of California San Diego, San Diego, California. 10University of Texas Southwestern Medical Center, Dallas, Texas.
11
Specialty Chair (Interventional Radiology), University of Chicago Hospital, Chicago, Illinois. 12Panel Chair (Vascular Imaging), UT Southwestern Medical
Center, Dallas.
The American College of Radiology seeks and encourages collaboration with other organizations on the development of the ACR Appropriateness
Criteria through society representation on expert panels. Participation by representatives from collaborating societies on the expert panel does not necessarily
imply individual or society endorsement of the final document.
Reprint requests to: publications@acr.org
ACR Appropriateness Criteria® 3 Chylothorax Treatment Planning
are at increased risk for postoperative adhesions, infection, and poor wound healing. Reported postoperative
mortality rates for patients who have failed conservative management range from 4.5% to as high as 50%
[2,4,9,10].
TDE is a percutaneous alternative to thoracic duct ligation. TDE allows for direct embolization (Type I) or needle
disruption of the thoracic duct (Type II). Whereas the former directly treats the focus of injury, the latter is
purported to create a controlled leak and inflammatory reaction in the retroperitoneum, which collateralizes and
diverts flow from the thoracic duct. Over several successive publications, Cope et al [14,15] defined the technique
and reported its feasibility. The initial series of 42 patients by Cope and Kaiser [16] revealed effective
percutaneous treatment in >70% of cases. In 109 patients with traumatic thoracic duct leak, Itkin et al [5] reported
90% clinical resolution postembolization and 72% clinical resolution of the chyle leak with thoracic duct
disruption. A subsequent report by Nadolski and Itkin [6] reported that TDE for nontraumatic chylous effusions in
34 patients was primarily successful if there was thoracic duct occlusion and extravasation. Pamarthi et al [7]
reported 85% technical success and 64% clinical success in 105 patients with all-cause chylous leaks. Additional
series have yielded similar results. Collectively, TDE has higher clinical success treating traumatic compared with
nontraumatic chyle leaks and with TDE compared with thoracic duct disruption [8,9,11,17]. Overall, acute
complications associated with TDE are minor and generally self-limited and are estimated at 2% to 6% [5-7].
Long-term complications may be seen in up to 14% of patients and may include leg swelling, abdominal swelling,
or chronic diarrhea [18].
Overview of Imaging Modalities
Different imaging studies serve different purposes in the evaluation and treatment of chylothorax.
Chest radiography
Chest radiographs are routine examinations to evaluate dyspnea, particularly in postoperative scenarios and in
patients who require intensive care. Radiographs can reliably detect pleural effusions or alternative diagnoses and
monitor the position of support lines and tubes [19]. Although there is a high sensitivity for pleural effusions, this
technique cannot reliably characterize the type of effusion.
Ultrasound
Ultrasound (US) is sensitive for the detection of pleural fluid but cannot definitively discriminate between the
types of pleural effusion [20]. US is now commonly used to help guide thoracentesis. Similarly, US can be used
to facilitate intranodal lymphangiography, which is becoming a more accepted technique. Beyond facilitating
these procedures, the role of US is limited with regard to the evaluation and management of chylothorax [21].
Conventional lymphangiography
Lymphangiography has historically been used to opacify lymphatic vessels, detect lymph nodes and metastatic
lesions, and evaluate lymphatic flow. Technological improvements in alternative diagnostic modalities led to an
abandonment of this technique for oncologic purposes because it was technically challenging and time intensive,
provided less information, and was more invasive. Although proficiency and training in the performance of
lymphangiograms decreased, the utility of lymphangiography to demonstrate lymphatic leak became an
established indication [8,9,14,22,23].
Traditionally, lymphangiography is performed from a pedal approach with the patient in a supine position. In this
technique, a dye such as methylene blue that stains the lymphatics is injected into the web spaces between the
toes. After the lymphatic vessel fills with the dye, the tissue overlying the lymphatic vessel is incised vertically,
the lymphatic vessel is carefully skeletonized, and a 30-gauge lymphangiography needle is used to access the
vessel. After securing the lymphatic access, 6 to 8 mL of ethiodized oil is instilled at a rate of 4 to 10 mL/h. Serial
spot radiographs from the foot to the chest are acquired approximately every 10 to 15 minutes to follow the
progression of the ethiodized oil as it ascends [22,24-27].
More recently, an interest in nodal lymphangiography has developed [21,28]. In this alternative approach, an
inguinal lymph node is targeted with a 25- to 26-gauge spinal needle under US guidance. The needle is positioned
between the hilum and cortex of a lymph node. Hand injection of ethiodized oil at a rate of 1 mL per 5 to 7
minutes is then initiated for a total volume of 6 to 10 mL. Serial spot radiographs of the pelvis, abdomen, and
thorax are then acquired to follow the progression of ethiodized oil [21,25,28-30]. Intranodal lymphangiography
appears to decrease procedure time, is less technically challenging, and decreases the risk of wound infection
when compared to pedal lymphangiography [21,28].
ACR Appropriateness Criteria® 4 Chylothorax Treatment Planning
Lymphangiography is able to define the site of the leak, diagnose alternative lymphatic vessel diseases, and
prevent unnecessary procedures. Several authors have documented the therapeutic benefit of lymphangiography
to occlude the site of leakage in 37% to 70% of patients without additional procedures [24-30]. Moreover, as
detailed earlier, lymphangiography is intrinsic to the performance of TDE and guides the transabdominal access
to the cisterna chyli and thoracic duct [14-16].
Nuclear lymphoscintigraphy
Nuclear lymphoscintigraphy images the pathways of lymphatic flow and lymph nodes and is most commonly
used to identify draining lymph nodes proximal to neoplasms. A few reports of lymphoscintigraphy with Tc-
99m–labeled radiotracers or orally administered iodine I-123-labeled 15-(4-iodophenyl)-3(R,S)-
methylpentadecanoic acid are present and demonstrate the potential to visualize the anatomic configuration of the
thoracic duct, reveal abnormal lymphatic drainage patterns, and potentially detect leaks [31-33]. However, aside
from a few small series, little is present in the literature to support its routine use in the diagnosis or treatment of
chylothorax.
Magnetic resonance imaging chest and abdomen
Visualization of the cisterna chyli, thoracic duct, and tributary lymphatic vessels with magnetic resonance
imaging (MRI) was described in healthy volunteers as early as 1999 [34]. Initial MR lymphangiography
technique involved unenhanced thin-collimated axial and coronal sequences similar to magnetic resonance
cholangiopancreatography. Further refinements of sequences, particularly heavily T2-weighted sequences with
and without and fat suppression, combined with 3-D techniques, maximum-intensity projections, and higher
magnetic fields, increased the reliability and quality of MR lymphangiography [35-39]. Morphological features of
the cisterna chyli and thoracic duct can be noted with identification of these structures in over 90% of
preoperative patients, potentially providing valuable information and decreasing their risk of lymphatic leak [40-
43].
The vast majority of studies are performed with unenhanced techniques, although delayed-phase cisterna chyli
enhancement has been noted [44]. Respiratory gating and further technical refinements have the potential to better
elucidate minor lymphatic vessels and lymphatic vessels in antidependent areas, which may not be seen through
conventional lymphangiography. Recent studies are beginning to document the feasibility of using gadolinium-
based contrast material injection within groin lymph nodes or in the web spaces between toes. Following the
contrast material injection, patients are imaged with MRI. High image quality of lymph nodes, central lymphatics,
and flow patterns within the lymphatics has been described, but these are preliminary research experiences and
are not widely available [45,46].
Computed tomography chest and abdomen
Older studies noted that noncontrast computed tomography (CT) visualizes the cisterna chyli in 1.7% of cases and
could differentiate this from adjacent anatomy by its low attenuation, continuity with the thoracic duct, and
tubular nature [47]. At least some portion of the thoracic duct was visualized in 55% of patients in a different
series [48].
Although MRI more reliably visualized more segments of the thoracic duct than CT, the addition of CT increased
the number of visualized segments [36]. More recent studies with 1-mm collimation and multiplanar reformation
were able to identify the thoracic duct and cisterna chyli in nearly 100% of CT scans with normal anatomy [49].
Older reports using a combination of lymphangiography and CT did not find any additional value of CT in
diagnosing the lymphatic injury, although in a more recent series, a combination of CT and unilateral pedal
lymphangiography was able to identify the cause and locate the leak in 75% of idiopathic chylothoraces after
failure of thoracic duct ligation [30]. Moreover, in this series of 24 patients, the lack of thoracic duct leakage was
managed with nonoperative therapy with higher success rates [30]. No evidence is present to suggest a role for
intravenous contrast material.
When the underlying etiology of chylothorax is unknown or nontraumatic, the speed, sensitivity, and specificity
of CT imaging can narrow the broader differential diagnosis.
Discussion of the Imaging Modalities by Variant
Variant 1: Chylothorax treatment planning: traumatic etiology.
Traumatic chylothoraces are a result of direct injury to thoracic lymphatics. Iatrogenic traumatic chylothorax
complicates up to 4% of esophageal resections [1,2,4-7]. Lung cancer resections, cardiovascular surgeries, and
ACR Appropriateness Criteria® 5 Chylothorax Treatment Planning
spinal surgeries can also be complicated by chylothorax, although at a lesser rate. Noniatrogenic causes of
traumatic chylothorax include penetrating trauma, fracture-dislocation of the spine, and hyperflexion injuries
[1,6,7]. Generally, the causative etiology is known in the traumatic setting. Sampling the pleural effusion
confirms the diagnosis of chylothorax. Imaging a patient with a known traumatic chylothorax serves only to
confirm the diagnosis and assist in therapeutic planning.
Chest radiography
In the setting of a traumatic injury to the thoracic duct, most commonly postoperative or mechanical trauma, chest
radiographs can confirm the presence of pleural fluid and lateralize the process and are routinely acquired in the
daily evaluation of supportive lines and tubes [19].
Ultrasound
US can be helpful in the guidance of thoracentesis and intranodal injection during lymphangiography [21].
Otherwise, US has little, if any, diagnostic role in the setting of a known traumatic chylothorax.
Conventional lymphangiography
Conventional lymphangiography is the gold standard for visualization of lymph nodes, lymphatic vessels, cisterna
chyli, the thoracic duct, and sites of injury [14,22,23]. Lymphangiography alone has been shown to be therapeutic
in a small percentage of patients, irrespective of attempts at TDE or disruption [24-27]. When performed as a
prelude to TDE, the combination is particularly effective in treating traumatic chylothorax, with technical and
clinical success rates approaching 90% [5-9,11].
Nuclear lymphoscintigraphy
Although nuclear lymphoscintigraphy may be able to confirm a lymphatic leak and identify the site, little
evidence is present to support its routine use [31-33]. Moreover, this adds little to the clinical care of a patient as
the traumatic etiology is usually known and any information gained would be redundant if conventional
lymphangiography was performed as part of TDE.
Magnetic resonance imaging chest and abdomen
The diagnostic benefit of MRI is negated in the setting of traumatic chylothoraces. However, the ability of MRI to
map the lymphatic system can be of benefit in select cases where identifying the cisterna chyli and/or thoracic
duct is difficult or conventional lymphangiography is unsuccessful [40-43].
Computed tomography chest and abdomen
CT imaging is able to visualize portions of lymphatic system but provides less anatomic detail than MRI
[36,47,48]. If the etiology is known, CT of the chest and abdomen, with or without intravenous contrast material,
has little value in that it does not help guide therapy directed at chylothorax in most cases.
Variant 2: Chylothorax treatment planning: nontraumatic or unknown etiology.
Nontraumatic chylothorax accounts for approximately 46% of chylothoraces and can be subcategorized as
resulting from malignancy, as occurs in 18% of all chylothoraces, or nonmalignant etiologies, which account for
28% of all chylothoraces [1,2,6,7]. Of the malignant etiologies, lymphoma is the leading cause, accounting for
75% of all malignant chylothoraces. Nonmalignant, nontraumatic chylothorax has been described in
lymphangioleiomyomatosis, sarcoidosis, cirrhosis, heart failure, nephrotic syndrome, venous thrombosis,
filariasis, venolymphatic malformations, and a variety of other congenital, idiopathic, and systemic diseases.
Approximately 9% of all chylous effusions are idiopathic [1,2,6,7]. Imaging a patient with either a nontraumatic
chylothorax or a chylothorax of unknown etiology serves to narrow the differential diagnosis, further characterize
the underlying cause and its severity, and assist in treatment planning.
Most patients with nontraumatic chylothoraces or chylothoraces of unknown etiologies present with acute
respiratory illness (ARI), which consists of 1 or more of the following: cough, sputum production, chest pain, or
dyspnea (with or without fever). The evaluation of ARI has been addressed by the American College of
Radiology (ACR), and the imaging evaluation includes chest radiography and chest CT [50,51]. The consistent
finding of chylothorax on initial imaging is the presence of a pleural effusion, which is a common medical
problem with more than 50 recognized causes [52]. Pleural fluid studies are necessary for definitive diagnosis and
to narrow the cause etiology of chylothorax.
ACR Appropriateness Criteria® 6 Chylothorax Treatment Planning
Chest radiography
Chest radiographs are routine examinations to evaluate dyspnea and have been designated as “usually
appropriate” in the workup of ARI. Radiographs can reliably detect pleural effusions or alternative diagnoses and
monitor the position of support lines and tubes [19,50,51]. This technique cannot reliably characterize the type of
effusion.
Ultrasound
US reliably detects pleural fluid but cannot definitively discriminate between the types of pleural effusion and
provides minimal additional information to narrow the differential diagnosis [20]. US can be helpful in the
guidance of thoracentesis and intranodal injection during lymphangiography [21].
Conventional lymphangiography
Conventional lymphangiography is the gold standard for visualization of lymph nodes, lymphatic vessels, cisterna
chyli, and the thoracic duct and for detection of lymphatic leakage [14,22,23]. In a nontraumatic or idiopathic
chylothorax, conventional lymphangiography may help diagnose lymphatic vessel diseases and anatomic
abnormalities and prevent unnecessary procedures. However, compared with traumatic chylothorax and
particularly in the setting of a systemic disease, conventional lymphangiography does not always elucidate the
underlying etiology. Additionally, TDE is less clinically effective in a nontraumatic chylothorax unless thoracic
duct occlusion or extravasation is present [6].
Nuclear lymphoscintigraphy
Nuclear lymphoscintigraphy has only a few reports that suggest it may be able to localize the site of chylous leak,
particularly if used with 3-D single-photon emission CT/CT techniques [31-33]. Scintigraphic imaging alone
provides limited localizing information and would not reliably narrow the differential diagnosis.
Magnetic resonance imaging chest and abdomen
MRI accurately visualizes lymphatic structures without intravenous contrast material, depicting abnormal
lymphatic malformations. With the addition of contrast material, MRI can characterize mediastinal masses,
pleural-based lesions, and chest wall pathology. However, thoracic MRI has limited utility for parenchymal lung
pathology [35-39].
Computed tomography chest and abdomen
Although CT imaging is inferior to MRI in visualizing lymphatics, it is a highly sensitive and specific
examination to narrow a broader differential diagnosis of thoracic and abdominal pathology [36,50,51].
Moreover, it is a rapid examination that is easily tolerated by a supine patient. Intravenous contrast material
accurately defines vascular and mediastinal structures and provides information on enhancement characteristics,
which is a consideration when the etiology of chylothorax is unknown.
Summary of Recommendations
• In traumatic chylothorax, chest radiographs are useful to confirm the presence of pleural fluid, lateralize the
process, and monitor the position of support lines and tubes. If further evaluation is warranted,
lymphangiography can precisely define the leak and offer therapeutic benefit, particularly if paired with TDE.
MRI and CT imaging are reserved for cases when lymphangiography is not diagnostic.
• Nontraumatic chylothorax can arise from a variety of disorders and may be a diagnostic dilemma. Chest
radiographs are useful to confirm the presence of pleural fluid and lateralize the process. MRI and CT
imaging are useful to narrow the differential diagnosis. Lymphangiography is helpful if a minimally invasive
approach to treatment is desired.
Summary of Evidence
Of the 52 references cited in the ACR Appropriateness Criteria® Chylothorax Treatment Planning document, 22
are categorized as therapeutic references, including 6 good-quality studies and 8 quality studies that may have
design limitations. Additionally, 30 references are categorized as diagnostic references, including 3 good-quality
studies and 5 quality studies that may have design limitations. There are 30 references that may not be useful as
primary evidence.
The 52 references cited in the ACR Appropriateness Criteria® Chylothorax Treatment Planning document were
published from 1989 through 2015.
ACR Appropriateness Criteria® 7 Chylothorax Treatment Planning
Although there are references that report on studies with design limitations, 9 good-quality studies provide good
evidence.
Relative Radiation Level Information
Potential adverse health effects associated with radiation exposure are an important factor to consider when
selecting the appropriate imaging procedure. Because there is a wide range of radiation exposures associated with
different diagnostic procedures, a relative radiation level (RRL) indication has been included for each imaging
examination. The RRLs are based on effective dose, which is a radiation dose quantity that is used to estimate
population total radiation risk associated with an imaging procedure. Patients in the pediatric age group are at
inherently higher risk from exposure, both because of organ sensitivity and longer life expectancy (relevant to the
long latency that appears to accompany radiation exposure). For these reasons, the RRL dose estimate ranges for
pediatric examinations are lower as compared to those specified for adults (see Table below). Additional
information regarding radiation dose assessment for imaging examinations can be found in the ACR
Appropriateness Criteria® Radiation Dose Assessment Introduction document.
Relative Radiation Level Designations
Adult Effective Dose Estimate Pediatric Effective Dose Estimate
Relative Radiation Level*
Range Range
O 0 mSv 0 mSv
☢ <0.1 mSv <0.03 mSv
☢☢ 0.1-1 mSv 0.03-0.3 mSv
☢☢☢ 1-10 mSv 0.3-3 mSv
☢☢☢☢ 10-30 mSv 3-10 mSv
☢☢☢☢☢ 30-100 mSv 10-30 mSv
*RRL assignments for some of the examinations cannot be made, because the actual patient doses in these procedures vary
as a function of a number of factors (eg, region of the body exposed to ionizing radiation, the imaging guidance that is
used). The RRLs for these examinations are designated as “Varies”.
Supporting Documents
For additional information on the Appropriateness Criteria methodology and other supporting documents go to
www.acr.org/ac.
References
1. Doerr CH, Allen MS, Nichols FC, 3rd, Ryu JH. Etiology of chylothorax in 203 patients. Mayo Clin Proc.
2005;80(7):867-870.
2. Maldonado F, Cartin-Ceba R, Hawkins FJ, Ryu JH. Medical and surgical management of chylothorax and
associated outcomes. Am J Med Sci. 2010;339(4):314-318.
3. Maldonado F, Hawkins FJ, Daniels CE, Doerr CH, Decker PA, Ryu JH. Pleural fluid characteristics of
chylothorax. Mayo Clin Proc. 2009;84(2):129-133.
4. Chen E, Itkin M. Thoracic duct embolization for chylous leaks. Semin Intervent Radiol. 2011;28(1):63-74.
5. Itkin M, Kucharczuk JC, Kwak A, Trerotola SO, Kaiser LR. Nonoperative thoracic duct embolization for
traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg. 2010;139(3):584-589;
discussion 589-590.
6. Nadolski GJ, Itkin M. Thoracic duct embolization for nontraumatic chylous effusion: experience in 34
patients. Chest. 2013;143(1):158-163.
7. Pamarthi V, Stecker MS, Schenker MP, et al. Thoracic duct embolization and disruption for treatment of
chylous effusions: experience with 105 patients. J Vasc Interv Radiol. 2014;25(9):1398-1404.
8. Boffa DJ, Sands MJ, Rice TW, et al. A critical evaluation of a percutaneous diagnostic and treatment strategy
for chylothorax after thoracic surgery. Eur J Cardiothorac Surg. 2008;33(3):435-439.
9. Lyon S, Mott N, Koukounaras J, Shoobridge J, Hudson PV. Role of interventional radiology in the
management of chylothorax: a review of the current management of high output chylothorax. Cardiovasc
Intervent Radiol. 2013;36(3):599-607.
10. Platis IE, Nwogu CE. Chylothorax. Thorac Surg Clin. 2006;16(3):209-214.
ACR Appropriateness Criteria® 8 Chylothorax Treatment Planning
11. Scorza LB, Goldstein BJ, Mahraj RP. Modern management of chylous leak following head and neck surgery:
a discussion of percutaneous lymphangiography-guided cannulation and embolization of the thoracic duct.
Otolaryngol Clin North Am. 2008;41(6):1231-1240, xi.
12. DePew ZS, Iqbal S, Mullon JJ, Nichols FC, Maldonado F. The role for tunneled indwelling pleural catheters
in patients with persistent benign chylothorax. Am J Med Sci. 2013;346(5):349-352.
13. Murphy MC, Newman BM, Rodgers BM. Pleuroperitoneal shunts in the management of persistent
chylothorax. Ann Thorac Surg. 1989;48(2):195-200.
14. Cope C. Diagnosis and treatment of postoperative chyle leakage via percutaneous transabdominal
catheterization of the cisterna chyli: a preliminary study. J Vasc Interv Radiol. 1998;9(5):727-734.
15. Cope C, Salem R, Kaiser LR. Management of chylothorax by percutaneous catheterization and embolization
of the thoracic duct: prospective trial. J Vasc Interv Radiol. 1999;10(9):1248-1254.
16. Cope C, Kaiser LR. Management of unremitting chylothorax by percutaneous embolization and blockage of
retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol. 2002;13(11):1139-1148.
17. Binkert CA, Yucel EK, Davison BD, Sugarbaker DJ, Baum RA. Percutaneous treatment of high-output
chylothorax with embolization or needle disruption technique. J Vasc Interv Radiol. 2005;16(9):1257-1262.
18. Laslett D, Trerotola SO, Itkin M. Delayed complications following technically successful thoracic duct
embolization. J Vasc Interv Radiol. 2012;23(1):76-79.
19. Amorosa JK, Bramwit MP, Mohammed TL, et al. ACR appropriateness criteria routine chest radiographs in
intensive care unit patients. J Am Coll Radiol. 2013;10(3):170-174.
20. Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hormann MF, Grabenwoger F. Quantification of
pleural effusions: sonography versus radiography. Radiology. 1994;191(3):681-684.
21. Nadolski GJ, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct
embolization. J Vasc Interv Radiol. 2012;23(5):613-616.
22. Kos S, Haueisen H, Lachmund U, Roeren T. Lymphangiography: forgotten tool or rising star in the diagnosis
and therapy of postoperative lymphatic vessel leakage. Cardiovasc Intervent Radiol. 2007;30(5):968-973.
23. Sachs PB, Zelch MG, Rice TW, Geisinger MA, Risius B, Lammert GK. Diagnosis and localization of
laceration of the thoracic duct: usefulness of lymphangiography and CT. AJR Am J Roentgenol.
1991;157(4):703-705.
24. Alejandre-Lafont E, Krompiec C, Rau WS, Krombach GA. Effectiveness of therapeutic lymphography on
lymphatic leakage. Acta Radiol. 2011;52(3):305-311.
25. Deso S, Ludwig B, Kabutey NK, Kim D, Guermazi A. Lymphangiography in the diagnosis and localization
of various chyle leaks. Cardiovasc Intervent Radiol. 2012;35(1):117-126.
26. Matsumoto T, Yamagami T, Kato T, et al. The effectiveness of lymphangiography as a treatment method for
various chyle leakages. Br J Radiol. 2009;82(976):286-290.
27. Ruan Z, Zhou Y, Wang S, Zhang J, Wang Y, Xu W. Clinical use of lymphangiography for intractable
spontaneous chylothorax. Thorac Cardiovasc Surg. 2011;59(7):430-435.
28. Rajebi MR, Chaudry G, Padua HM, et al. Intranodal lymphangiography: feasibility and preliminary
experience in children. J Vasc Interv Radiol. 2011;22(9):1300-1305.
29. Kawasaki R, Sugimoto K, Fujii M, et al. Therapeutic effectiveness of diagnostic lymphangiography for
refractory postoperative chylothorax and chylous ascites: correlation with radiologic findings and preceding
medical treatment. AJR Am J Roentgenol. 2013;201(3):659-666.
30. Liu DY, Shao Y, Shi JX. Unilateral pedal lymphangiography with non-contrast computerized tomography is
valuable in the location and treatment decision of idiopathic chylothorax. J Cardiothorac Surg. 2014;9:8.
31. Pui MH, Yueh TC. Lymphoscintigraphy in chyluria, chyloperitoneum and chylothorax. J Nucl Med.
1998;39(7):1292-1296.
32. Takanami K, Ichikawa H, Fukuda H, Takahashi S. Three-dimensional lymphoscintigraphy using SPECT/CT
and 123I-BMIPP for the preoperative detection of anatomical anomalies of the thoracic duct. Clin Nucl Med.
2012;37(11):1047-1051.
33. Yang J, Codreanu I, Zhuang H. Minimal lymphatic leakage in an infant with chylothorax detected by
lymphoscintigraphy SPECT/CT. Pediatrics. 2014;134(2):e606-610.
34. Hayashi S, Miyazaki M. Thoracic duct: visualization at nonenhanced MR lymphography--initial experience.
Radiology. 1999;212(2):598-600.
35. Erden A, Fitoz S, Yagmurlu B, Erden I. Abdominal confluence of lymph trunks: detectability and
morphology on heavily T2-weighted images. AJR Am J Roentgenol. 2005;184(1):35-40.
ACR Appropriateness Criteria® 9 Chylothorax Treatment Planning
36. Kato T, Takase K, Ichikawa H, Satomi S, Takahashi S. Thoracic duct visualization: combined use of
multidetector-row computed tomography and magnetic resonance imaging. J Comput Assist Tomogr.
2011;35(2):260-265.
37. Matsushima S, Ichiba N, Hayashi D, Fukuda K. Nonenhanced magnetic resonance lymphoductography:
visualization of lymphatic system of the trunk on 3-dimensional heavily T2-weighted image with 2-
dimensional prospective acquisition and correction. J Comput Assist Tomogr. 2007;31(2):299-302.
38. Pinto PS, Sirlin CB, Andrade-Barreto OA, Brown MA, Mindelzun RE, Mattrey RF. Cisterna chyli at routine
abdominal MR imaging: a normal anatomic structure in the retrocrural space. Radiographics. 2004;24(3):809-
817.
39. Takahashi H, Kuboyama S, Abe H, Aoki T, Miyazaki M, Nakata H. Clinical feasibility of noncontrast-
enhanced magnetic resonance lymphography of the thoracic duct. Chest. 2003;124(6):2136-2142.
40. Okuda I, Udagawa H, Hirata K, Nakajima Y. Depiction of the thoracic duct by magnetic resonance imaging:
comparison between magnetic resonance imaging and the anatomical literature. Jpn J Radiol. 2011;29(1):39-
45.
41. Okuda I, Udagawa H, Takahashi J, Yamase H, Kohno T, Nakajima Y. Magnetic resonance-thoracic
ductography: imaging aid for thoracic surgery and thoracic duct depiction based on embryological
considerations. Gen Thorac Cardiovasc Surg. 2009;57(12):640-646.
42. Yu DX, Ma XX, Wang Q, Zhang Y, Li CF. Morphological changes of the thoracic duct and accessory
lymphatic channels in patients with chylothorax: detection with unenhanced magnetic resonance imaging. Eur
Radiol. 2013;23(3):702-711.
43. Yu DX, Ma XX, Zhang XM, Wang Q, Li CF. Morphological features and clinical feasibility of thoracic duct:
detection with nonenhanced magnetic resonance imaging at 3.0 T. J Magn Reson Imaging. 2010;32(1):94-
100.
44. Verma SK, Mitchell DG, Bergin D, Mehta R, Chopra S, Choi D. The cisterna chyli: enhancement on delayed
phase MR images after intravenous administration of gadolinium chelate. Radiology. 2007;244(3):791-796.
45. Krishnamurthy R, Hernandez A, Kavuk S, Annam A, Pimpalwar S. Imaging the central conducting
lymphatics: initial experience with dynamic MR lymphangiography. Radiology. 2015;274(3):871-878.
46. Liu NF, Lu Q, Jiang ZH, Wang CG, Zhou JG. Anatomic and functional evaluation of the lymphatics and
lymph nodes in diagnosis of lymphatic circulation disorders with contrast magnetic resonance
lymphangiography. J Vasc Surg. 2009;49(4):980-987.
47. Smith TR, Grigoropoulos J. The cisterna chyli: incidence and characteristics on CT. Clin Imaging.
2002;26(1):18-22.
48. Liu ME, Branstetter BFt, Whetstone J, Escott EJ. Normal CT appearance of the distal thoracic duct. AJR Am J
Roentgenol. 2006;187(6):1615-1620.
49. Kiyonaga M, Mori H, Matsumoto S, Yamada Y, Sai M, Okada F. Thoracic duct and cisterna chyli: evaluation
with multidetector row CT. Br J Radiol. 2012;85(1016):1052-1058.
50. American College of Radiology. ACR Appropriateness Criteria®: Acute Respiratory Illness in
Immunocompromised Patients. Available at: https://acsearch.acr.org/docs/69447/Narrative/.
51. American College of Radiology. ACR Appropriateness Criteria®: Acute Respiratory Illness in
Immunocompetent Patients. Available at: https://acsearch.acr.org/docs/69446/Narrative/.
52. Hooper C, Lee YC, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic
Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii4-17.
The ACR Committee on Appropriateness Criteria and its expert panels have developed criteria for determining appropriate imaging examinations for
diagnosis and treatment of specified medical condition(s). These criteria are intended to guide radiologists, radiation oncologists and referring physicians
in making decisions regarding radiologic imaging and treatment. Generally, the complexity and severity of a patient’s clinical condition should dictate the
selection of appropriate imaging procedures or treatments. Only those examinations generally used for evaluation of the patient’s condition are ranked.
Other imaging studies necessary to evaluate other co-existent diseases or other medical consequences of this condition are not considered in this
document. The availability of equipment or personnel may influence the selection of appropriate imaging procedures or treatments. Imaging techniques
classified as investigational by the FDA have not been considered in developing these criteria; however, study of new equipment and applications should
be encouraged. The ultimate decision regarding the appropriateness of any specific radiologic examination or treatment must be made by the referring
physician and radiologist in light of all the circumstances presented in an individual examination.
ACR Appropriateness Criteria® 10 Chylothorax Treatment Planning
You might also like
- Indeterminate Renal Mass ACR Appropriateness CriteriaDocument10 pagesIndeterminate Renal Mass ACR Appropriateness CriteriaAndrew TaliaferroNo ratings yet
- Onset Flank PainDocument11 pagesOnset Flank PainPipitNo ratings yet
- Renal Cell Carcinoma Staging (ACR Appropriateness Criteria)Document10 pagesRenal Cell Carcinoma Staging (ACR Appropriateness Criteria)Andrew TaliaferroNo ratings yet
- Ca - Treatment-Breast-Inflammatory-Web-AlgorithmDocument19 pagesCa - Treatment-Breast-Inflammatory-Web-AlgorithmAgustin SierraNo ratings yet
- American College of Radiology ACR Appropriateness CriteriaDocument7 pagesAmerican College of Radiology ACR Appropriateness CriteriaMeutia PutriNo ratings yet
- American College of Radiology End User License AgreementDocument9 pagesAmerican College of Radiology End User License AgreementYori PrimandaNo ratings yet
- PDFsam ObGynDocument4 pagesPDFsam ObGynAlessandro ZadraNo ratings yet
- Acute PancreatitisDocument16 pagesAcute PancreatitisLoredana BoghezNo ratings yet
- Abdominal Aortic Aneurysm Follow-Up (Without Repair)Document7 pagesAbdominal Aortic Aneurysm Follow-Up (Without Repair)victor contrerasNo ratings yet
- Postmenopausal Subacute or Chronic Pelvic PainDocument9 pagesPostmenopausal Subacute or Chronic Pelvic PainM SNo ratings yet
- Rationale Diagnostic ProceduresDocument22 pagesRationale Diagnostic ProceduresShaktisila FatrahadyNo ratings yet
- Diagnostic Imaging of Breast: Radiology DepartementDocument35 pagesDiagnostic Imaging of Breast: Radiology DepartementAnonymous 9NC74wAL1No ratings yet
- Acute Onset Flank Pain-Suspicion of Stone DiseaseDocument9 pagesAcute Onset Flank Pain-Suspicion of Stone DiseasecubirojasNo ratings yet
- Adrenal Mass EvaluationDocument19 pagesAdrenal Mass Evaluationpj rakNo ratings yet
- American College of Radiology ACR Appropriateness CriteriaDocument10 pagesAmerican College of Radiology ACR Appropriateness CriteriaNovia ChrisnawatiNo ratings yet
- Hematuria PDFDocument11 pagesHematuria PDFPipitNo ratings yet
- Tecnologias Preventivaz - ActENF - Clinica - CompressedDocument2 pagesTecnologias Preventivaz - ActENF - Clinica - Compressedadrianjv04No ratings yet
- Breast PainDocument9 pagesBreast PainmarysrbNo ratings yet
- Apendicectomia LaparoscopicaDocument6 pagesApendicectomia LaparoscopicaCirugiapediatrica LaRazaNo ratings yet
- Page 1 of 5: Guidelines/green-Top-Guidelines/gtg - 67 - Endometrial - Hyperplasia PDFDocument5 pagesPage 1 of 5: Guidelines/green-Top-Guidelines/gtg - 67 - Endometrial - Hyperplasia PDFfitrah fajrianiNo ratings yet
- Breast Implant Evaluation - NarrativeDocument25 pagesBreast Implant Evaluation - Narrativeopenlab.doisNo ratings yet
- ACR Appropriateness Criteria For HematuriaDocument8 pagesACR Appropriateness Criteria For HematuriaAndrew TaliaferroNo ratings yet
- Case SurgeryDocument5 pagesCase SurgeryJayzelle Anne de LeonNo ratings yet
- Blunt Abdominal TraumaDocument11 pagesBlunt Abdominal TraumaRahmat SyahiliNo ratings yet
- ACR Appropriateness Criteria® Acute Chest Pain - Suspected Pulmonary Embolism.Document16 pagesACR Appropriateness Criteria® Acute Chest Pain - Suspected Pulmonary Embolism.Madalina TalpauNo ratings yet
- Radiologic Management of Upper Gastrointestinal BleedingDocument13 pagesRadiologic Management of Upper Gastrointestinal BleedingshadowlightfoxNo ratings yet
- Seminars in Pediatric Surgery: Laparoscopic Vertical Sleeve Gastrectomy For Adolescents With Morbid ObesityDocument3 pagesSeminars in Pediatric Surgery: Laparoscopic Vertical Sleeve Gastrectomy For Adolescents With Morbid ObesityDr Venkatachalapathy T S Ped SurgeonNo ratings yet
- Jourding Endometriosis NICEDocument36 pagesJourding Endometriosis NICEbudi haryadiNo ratings yet
- CS-PUB-LAB-1 Cervical Cytology Management Recommendations Explanatory GuideDocument11 pagesCS-PUB-LAB-1 Cervical Cytology Management Recommendations Explanatory GuideMc Coral100% (1)
- Abdominal Aortic Aneurysm-Interventional Planning and Follow-UpDocument13 pagesAbdominal Aortic Aneurysm-Interventional Planning and Follow-Upvictor contrerasNo ratings yet
- A Rare Case of Multiple Ileal Lipoma in A Young MaleDocument4 pagesA Rare Case of Multiple Ileal Lipoma in A Young MaleAthenaeum Scientific PublishersNo ratings yet
- Ca Treatment Rectal Web AlgorithmDocument17 pagesCa Treatment Rectal Web AlgorithmPatrick JohnNo ratings yet
- Acute Respiratory Illness in Immunocompetent PatientsDocument14 pagesAcute Respiratory Illness in Immunocompetent PatientsVianney OlveraNo ratings yet
- Health Talk On BSFDocument11 pagesHealth Talk On BSFjyoti singh100% (1)
- FMcase 1Document24 pagesFMcase 1Ryan Townsend100% (2)
- Ca Treatment Breast Noninvasive Web AlgorithmDocument8 pagesCa Treatment Breast Noninvasive Web AlgorithmAgustin SierraNo ratings yet
- Masa Palpable en MamaDocument22 pagesMasa Palpable en MamaarcaNo ratings yet
- Abdominal Truama (Handout)Document6 pagesAbdominal Truama (Handout)Ayuub AbdirizakNo ratings yet
- Acute Nonlocalized Abdominal PainDocument17 pagesAcute Nonlocalized Abdominal PainghassanNo ratings yet
- Guidelines On Clinical Management of Endometrial HyperplasiaDocument14 pagesGuidelines On Clinical Management of Endometrial Hyperplasiaمريم مريمNo ratings yet
- Examination of The Breast: 11/14/2011 © Clinical Skills Resource Centre, University of Liverpool, UKDocument19 pagesExamination of The Breast: 11/14/2011 © Clinical Skills Resource Centre, University of Liverpool, UKNilakshi Barik MandalNo ratings yet
- Distopia PDFDocument10 pagesDistopia PDFKevin Anthony Villanueva PaucarNo ratings yet
- Occupational Lung DiseasesDocument13 pagesOccupational Lung DiseasesVianney OlveraNo ratings yet
- Examination of The BreastDocument10 pagesExamination of The BreastHina MalikNo ratings yet
- Diagnostic Modalities in Blunt Abdominal Trauma: Diagnostic Peritoneal Lavage (DPL) CAT Scan Ultrasound (FAST Exam)Document52 pagesDiagnostic Modalities in Blunt Abdominal Trauma: Diagnostic Peritoneal Lavage (DPL) CAT Scan Ultrasound (FAST Exam)Tenobella AnggrainiNo ratings yet
- Breast LectureDocument98 pagesBreast LectureTopher ReyesNo ratings yet
- Abdominoplasty Combined With Cesarean DeliveryDocument7 pagesAbdominoplasty Combined With Cesarean Deliverymahmoud makramNo ratings yet
- Laparoscopic Atypical Gastrectomy For Symptomatic Gastric LipomaDocument5 pagesLaparoscopic Atypical Gastrectomy For Symptomatic Gastric LipomaMaghfirah MahmuddinNo ratings yet
- Abnormal Uterine BleedingDocument12 pagesAbnormal Uterine Bleedingvictor contrerasNo ratings yet
- Do We Need Anorectal Physiology Work Up in The Daily Colorectal Praxis?Document6 pagesDo We Need Anorectal Physiology Work Up in The Daily Colorectal Praxis?soudrackNo ratings yet
- College of Medicine and Health Sciences: For Public Health C-I StudentsDocument29 pagesCollege of Medicine and Health Sciences: For Public Health C-I StudentsWĩž BŕëãžÿNo ratings yet
- Pezner 2015Document5 pagesPezner 2015drrajeshb77No ratings yet
- GTG 67 Endometrial HyperplasiaDocument31 pagesGTG 67 Endometrial HyperplasiaFinka Inarae100% (1)
- Protocol For Blunt Abdominal TraumaDocument19 pagesProtocol For Blunt Abdominal TraumaabigailNo ratings yet
- Total Laparoscopic HysterectomyDocument8 pagesTotal Laparoscopic HysterectomySamir CastilloNo ratings yet
- Penetrating Abdominal TraumaDocument3 pagesPenetrating Abdominal TraumaamrulNo ratings yet
- Case 1 49y.o. With Medical Algorithm - Surgery 2 Juanillo - 12.01.15Document6 pagesCase 1 49y.o. With Medical Algorithm - Surgery 2 Juanillo - 12.01.15นีล ไบรอันNo ratings yet
- 0102 6720 Abcd 32 01 E1426Document5 pages0102 6720 Abcd 32 01 E1426Amaury de la CruzNo ratings yet
- Pap SmearDocument32 pagesPap SmearAmira Nasir100% (1)
- Hysterectomy, (Removal of Uterus) A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandHysterectomy, (Removal of Uterus) A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsRating: 5 out of 5 stars5/5 (1)
- Acute Respiratory Illness in Immunocompetent PatientsDocument14 pagesAcute Respiratory Illness in Immunocompetent PatientsVianney OlveraNo ratings yet
- Occupational Lung DiseasesDocument13 pagesOccupational Lung DiseasesVianney OlveraNo ratings yet
- Pneumonia in The Immunocompetent Child ETDocument32 pagesPneumonia in The Immunocompetent Child ETVianney OlveraNo ratings yet
- Radiologic Management of Thoracic Nodules and MassesDocument14 pagesRadiologic Management of Thoracic Nodules and MassesVianney OlveraNo ratings yet
- ABARI-Volunteer Guide BookDocument10 pagesABARI-Volunteer Guide BookEla Mercado0% (1)
- PM Jobs Comp Ir RandDocument9 pagesPM Jobs Comp Ir Randandri putrantoNo ratings yet
- Instant Download Business in Action 7Th Edition Bovee Solutions Manual PDF ScribdDocument17 pagesInstant Download Business in Action 7Th Edition Bovee Solutions Manual PDF ScribdLance CorreaNo ratings yet
- Iguard® LM SeriesDocument82 pagesIguard® LM SeriesImran ShahidNo ratings yet
- Universal Ultrasonic Generator For Welding: W. Kardy, A. Milewski, P. Kogut and P. KlukDocument3 pagesUniversal Ultrasonic Generator For Welding: W. Kardy, A. Milewski, P. Kogut and P. KlukPhilip EgyNo ratings yet
- John DrydenDocument3 pagesJohn DrydenDunas SvetlanaNo ratings yet
- Niveshdaily: From Research DeskDocument53 pagesNiveshdaily: From Research DeskADNo ratings yet
- Grammar and Vocabulary TestDocument5 pagesGrammar and Vocabulary TestLeonora ConejosNo ratings yet
- Lady in The House, Her Responsibilities & Ambitions: Amrita DuhanDocument7 pagesLady in The House, Her Responsibilities & Ambitions: Amrita DuhanFitness FableNo ratings yet
- Registration ListDocument5 pagesRegistration ListGnanesh Shetty BharathipuraNo ratings yet
- Sample CVFormat 1Document2 pagesSample CVFormat 1subham.sharmaNo ratings yet
- Soft Skills & Personality DevelopmentDocument62 pagesSoft Skills & Personality DevelopmentSajid PashaNo ratings yet
- Lab 3 Arduino Led Candle Light: CS 11/group - 4 - Borromeo, Galanida, Pabilan, Paypa, TejeroDocument3 pagesLab 3 Arduino Led Candle Light: CS 11/group - 4 - Borromeo, Galanida, Pabilan, Paypa, TejeroGladys Ruth PaypaNo ratings yet
- Arithmetic QuestionsDocument2 pagesArithmetic QuestionsAmir KhanNo ratings yet
- Caterpillar Cat C7 Marine Engine Parts Catalogue ManualDocument21 pagesCaterpillar Cat C7 Marine Engine Parts Catalogue ManualkfsmmeNo ratings yet
- Python in Hidrology BookDocument153 pagesPython in Hidrology BookJuan david Gonzalez vasquez100% (1)
- Philodendron Plants CareDocument4 pagesPhilodendron Plants CareSabre FortNo ratings yet
- Lodge at The Ancient City Information Kit / Great ZimbabweDocument37 pagesLodge at The Ancient City Information Kit / Great ZimbabwecitysolutionsNo ratings yet
- SP-Chapter 14 PresentationDocument83 pagesSP-Chapter 14 PresentationLoiDa FloresNo ratings yet
- Trina 440W Vertex-S+ DatasheetDocument2 pagesTrina 440W Vertex-S+ DatasheetBrad MannNo ratings yet
- (20836104 - Artificial Satellites) Investigation of The Accuracy of Google Earth Elevation DataDocument9 pages(20836104 - Artificial Satellites) Investigation of The Accuracy of Google Earth Elevation DataSunidhi VermaNo ratings yet
- Breastfeeding W Success ManualDocument40 pagesBreastfeeding W Success ManualNova GaveNo ratings yet
- Img 20150510 0001Document2 pagesImg 20150510 0001api-284663984No ratings yet
- USER MANUAL ABRITES Commander For Nissan PDFDocument20 pagesUSER MANUAL ABRITES Commander For Nissan PDFBosi GashiNo ratings yet
- Quality Control of Rigid Pavements 1Document58 pagesQuality Control of Rigid Pavements 1pranjpatil100% (1)
- Contemporary Strategic ManagementDocument2 pagesContemporary Strategic ManagementZee Dee100% (1)
- 4.2.4.5 Packet Tracer - Connecting A Wired and Wireless LAN InstructionsDocument5 pages4.2.4.5 Packet Tracer - Connecting A Wired and Wireless LAN InstructionsAhmadHijaziNo ratings yet
- MFE Module 1 .Document15 pagesMFE Module 1 .Adarsh KNo ratings yet
- Federalist Papers 10 51 ExcerptsDocument2 pagesFederalist Papers 10 51 Excerptsapi-292351355No ratings yet
- BiografijaDocument36 pagesBiografijaStjepan ŠkalicNo ratings yet