Professional Documents
Culture Documents
U5 - R3 - Osmosis Worksheet
Uploaded by
poonamOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
U5 - R3 - Osmosis Worksheet
Uploaded by
poonamCopyright:
Available Formats
Gurgaon
2022-23
Name: Grade VII _ Subject: Expedition (Osmosis and Diffusion) Date:
_________________________________________________________________________________________
Learning Target: I can explain how kidneys maintain the amount of water in the body.
I can explain the differences between Osmosis and Diffusion
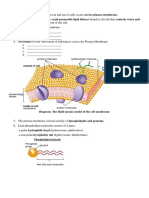
Task: In the images given below raisins placed in beakers of various concentrations. Observe the images carefully
and draw an arrow to show which way the water would move by Osmosis
water water water
water water water
water water water
water water water
water
Osmosis and Diffusion
Reader’s
. purpose:
Explain the differences between Osmosis and Diffusion
Osmosis: Osmosis is the movement of solvent particles across a semipermeable membrane from a dilute
solution into a concentrated solution. The solvent moves to dilute the concentrated solution and equalize the
concentration on both sides of the membrane.
Examples of Osmosis:
Red blood cells swelling up when exposed to freshwater and plant root hairs taking up water.
To see an easy demonstration of osmosis, soak gummy candies in water. The gel of the candies
acts as a semipermeable membrane.
Diffusion: Diffusion is the movement of particles from an area of higher concentration to lower concentration.
The overall effect is to equalize concentration throughout the medium.
Examples of Diffusion:
The scent of perfume filling a whole room and the movement of small molecules across a cell
membrane.
Adding a drop of food coloring to water
Osmosis and diffusion are related processes, as both processes equalize the concentration of two solutions.
Both are passive transport processes, which means they do not require any input of extra energy to occur.
Task: Write the differences between Osmosis and Diffusion in the table below
Osmosis Diffusion
You might also like
- Lesson Plan SKELETAL SYSTEMDocument11 pagesLesson Plan SKELETAL SYSTEMpoonamNo ratings yet
- Assessment ElectricityDocument3 pagesAssessment ElectricitypoonamNo ratings yet
- Chapter 3 - Diffusion & OsmosisDocument26 pagesChapter 3 - Diffusion & Osmosisapi-3728508100% (6)
- Reverse Osmosis Treatment of Drinking WaterFrom EverandReverse Osmosis Treatment of Drinking WaterRating: 3.5 out of 5 stars3.5/5 (4)
- Diffusion and Osmosis WorksheetDocument2 pagesDiffusion and Osmosis Worksheetganesh mukhiaNo ratings yet
- O Level - Osmosis and DiffusionDocument12 pagesO Level - Osmosis and DiffusionArif UllahNo ratings yet
- Slurry HDPE Process TechnologyDocument18 pagesSlurry HDPE Process TechnologyGenilson E Neliane Silva100% (1)
- Chem123 Lab Notes PrelimDocument1 pageChem123 Lab Notes PrelimKristine PangahinNo ratings yet
- Preview Test 3 Reading: Passage 1Document8 pagesPreview Test 3 Reading: Passage 1wolan hariyantoNo ratings yet
- Diffusion: The More Particles There Are in A Certain Volume, The More Concentrated Those Particles AreDocument6 pagesDiffusion: The More Particles There Are in A Certain Volume, The More Concentrated Those Particles AreGia Espinosa OcbeñaNo ratings yet
- Lab Sheet 1Document2 pagesLab Sheet 1MELISSA NANONGNo ratings yet
- Section 8.2 PG 297Document11 pagesSection 8.2 PG 297Ethan RajakumarNo ratings yet
- Mariam Toma Biology Micro Teaching - Osmosis and DiffusionDocument19 pagesMariam Toma Biology Micro Teaching - Osmosis and DiffusionMariam AmgadNo ratings yet
- U5 - R2 - Working of KidneysDocument2 pagesU5 - R2 - Working of KidneyspoonamNo ratings yet
- WEEK 002 Intermolecular Forces of Matter and Properties of LiquidsDocument7 pagesWEEK 002 Intermolecular Forces of Matter and Properties of LiquidsBleep BloopNo ratings yet
- Reminder B3Document9 pagesReminder B3Daniel thanh ducNo ratings yet
- Diffusion Lab Docx 1Document12 pagesDiffusion Lab Docx 1api-319962139No ratings yet
- Diffusion and Osmosis - IGCSEDocument37 pagesDiffusion and Osmosis - IGCSEJohnnie ZhangNo ratings yet
- Difference Between Osmosis and DiffusionDocument9 pagesDifference Between Osmosis and DiffusionSol SolNo ratings yet
- Reverse OsmosisDocument3 pagesReverse OsmosisJOSEPH HERBERT MABELNo ratings yet
- Osmosis PPT 2Document4 pagesOsmosis PPT 2api-309893409No ratings yet
- 3 Diffusion and Osmosis Protocol - Spring 2014Document12 pages3 Diffusion and Osmosis Protocol - Spring 2014ryandakotaNo ratings yet
- Biology Exercise 3/ex - Transportacrossmembrane/Ymt/Aoghss Name: - 4DDocument2 pagesBiology Exercise 3/ex - Transportacrossmembrane/Ymt/Aoghss Name: - 4DSuki LeungNo ratings yet
- Bio3 TransiDocument47 pagesBio3 TransichemasuetNo ratings yet
- Movement of Substances Rev Sci Yr7Document4 pagesMovement of Substances Rev Sci Yr7farhan.18sepNo ratings yet
- MODULE 3 - Group 4 PDFDocument7 pagesMODULE 3 - Group 4 PDFAnne Therese / Annie KanaanNo ratings yet
- Topic-Osmosis, Reverse Osmosis and Its Applications: Chemistry Investigatory ProjectDocument21 pagesTopic-Osmosis, Reverse Osmosis and Its Applications: Chemistry Investigatory ProjectAmmar Adil100% (1)
- W1 M3-E-text-Introduction To Hydrodynamic TechniquesDocument10 pagesW1 M3-E-text-Introduction To Hydrodynamic TechniquesRoahit RajanNo ratings yet
- Lab 02 Diffusion Osmosis PermeabilityDocument18 pagesLab 02 Diffusion Osmosis Permeabilitydorelia_simonaNo ratings yet
- Lab 1 - Osmosis & Diffusion - AU21Document10 pagesLab 1 - Osmosis & Diffusion - AU21Ava ChappellNo ratings yet
- This Study Resource Was: General Chemistry 2 Answer SheetDocument4 pagesThis Study Resource Was: General Chemistry 2 Answer SheetYuan Andrei SantosNo ratings yet
- Diffusion and OsmosisDocument3 pagesDiffusion and OsmosisRenee Dwi Permata MessakaraengNo ratings yet
- AP Biology Lab 2 Osmosis and DiffusionDocument8 pagesAP Biology Lab 2 Osmosis and DiffusionSourav ChakrabortyNo ratings yet
- Diffusion Osmosis pt2Document20 pagesDiffusion Osmosis pt2api-423980580No ratings yet
- Osmosis NotesDocument3 pagesOsmosis NotessmexiiloriNo ratings yet
- 5-Mechanisms of Active and Pasive Transport Through TheDocument61 pages5-Mechanisms of Active and Pasive Transport Through Thec3rberussNo ratings yet
- 13 Hard Starch Czech Republic Klara Rozenkova 82-85 Proc 18th IYPT 2005Document4 pages13 Hard Starch Czech Republic Klara Rozenkova 82-85 Proc 18th IYPT 2005YM YenNo ratings yet
- Biology Chapter 1Document7 pagesBiology Chapter 1Kuan LoongNo ratings yet
- Transport Across MembraneDocument5 pagesTransport Across MembraneRena Emmanuelle PalenciaNo ratings yet
- Notes - Movement of SubstancesDocument8 pagesNotes - Movement of SubstancesEricNo ratings yet
- Osmosis: Diffusion Is The Process Where Molecules of Two or More Substances Move AboutDocument3 pagesOsmosis: Diffusion Is The Process Where Molecules of Two or More Substances Move AboutizzahNo ratings yet
- 06-Movement of Materials-1Document11 pages06-Movement of Materials-1Maku MichaelNo ratings yet
- Gen Chem 2 L3.1 Properties of LiquidsDocument6 pagesGen Chem 2 L3.1 Properties of LiquidsrenieljrebuengaNo ratings yet
- Chemistry Lab Report 3Document6 pagesChemistry Lab Report 3Jinyoung KimNo ratings yet
- LESSON 5 Genchem2Document20 pagesLESSON 5 Genchem2J NavarroNo ratings yet
- Water RelationDocument62 pagesWater RelationBlister CountNo ratings yet
- Alea - Exercise 7 Cellular Transport MechanismsDocument4 pagesAlea - Exercise 7 Cellular Transport MechanismsChristine AleaNo ratings yet
- Viscosity ActivityDocument2 pagesViscosity ActivityYvanna CaigoyNo ratings yet
- Kami Export - Experiment 1Document9 pagesKami Export - Experiment 1MAYNARD E. ABONADORNo ratings yet
- Diffusion Vs OsmosisDocument3 pagesDiffusion Vs OsmosisK TanNo ratings yet
- Intorductory of Crop Physiology Water Arrangement of Water MoleculesDocument17 pagesIntorductory of Crop Physiology Water Arrangement of Water MoleculesSHADOW GAMINGNo ratings yet
- Environmental Engineering Project: OsmosisDocument18 pagesEnvironmental Engineering Project: OsmosisKeerthiNo ratings yet
- Water Potential and OsmosisDocument16 pagesWater Potential and OsmosisSơn Minh LươngNo ratings yet
- Fluid Mechanics Prelim 2Document4 pagesFluid Mechanics Prelim 2Santa mariaNo ratings yet
- Maronga Chri ch311 Ass1Document5 pagesMaronga Chri ch311 Ass1L3WIS J CHIHURINo ratings yet
- OsmosisDocument19 pagesOsmosisdamyangdcsNo ratings yet
- ProjectDocument18 pagesProjectSmruti Ranjan MallaNo ratings yet
- Potential, Which Is A Component of The Driving Force of Water Movement inDocument4 pagesPotential, Which Is A Component of The Driving Force of Water Movement inKat HanyaneNo ratings yet
- Plant Physiolog-WPS OfficeDocument45 pagesPlant Physiolog-WPS OfficeShubhendu ChattopadhyayNo ratings yet
- Dye Penetrant Testing Q&ADocument5 pagesDye Penetrant Testing Q&AHarry FrankNo ratings yet
- Exploring Properties of Liquids: Activity #1: Moving WaterDocument2 pagesExploring Properties of Liquids: Activity #1: Moving WaterJoana Mae Balilia DiazNo ratings yet
- Diffusion OsmosisDocument20 pagesDiffusion OsmosisWing PangNo ratings yet
- Document For VideosDocument1 pageDocument For VideospoonamNo ratings yet
- Q1. HI!! I Am Joe's Femur, Once Again. Good To See You All. Read The Excerpt From Joe's Body's Biography and Answer The QuestionsDocument4 pagesQ1. HI!! I Am Joe's Femur, Once Again. Good To See You All. Read The Excerpt From Joe's Body's Biography and Answer The QuestionspoonamNo ratings yet
- DLP - Urinary SystemDocument6 pagesDLP - Urinary SystempoonamNo ratings yet
- Urinary System ReadingDocument4 pagesUrinary System ReadingpoonamNo ratings yet
- Learningtarget:: Activity 1 ADocument4 pagesLearningtarget:: Activity 1 ApoonamNo ratings yet
- ExcretoryDocument3 pagesExcretorypoonamNo ratings yet
- URINARY SYSTEM - Autonomous ModuleDocument9 pagesURINARY SYSTEM - Autonomous ModulepoonamNo ratings yet
- Final Day Urinary SystemDocument17 pagesFinal Day Urinary SystempoonamNo ratings yet
- Lesson Plan RocksDocument6 pagesLesson Plan RockspoonamNo ratings yet
- Fluid Problem SetDocument4 pagesFluid Problem SetJupissa Espinosa100% (1)
- Equivalent Weight DeterminationDocument9 pagesEquivalent Weight DeterminationJohnNo ratings yet
- Lindsay Et Al, 1959 IIIDocument4 pagesLindsay Et Al, 1959 IIIexportar2299No ratings yet
- Homework #1 For Chemical Reaction Engineering IIDocument2 pagesHomework #1 For Chemical Reaction Engineering IIRaushan KumarNo ratings yet
- Ex.2 CHM 3 Sec.1 Fall 2020 (PRB.)Document3 pagesEx.2 CHM 3 Sec.1 Fall 2020 (PRB.)Sn CarbonelNo ratings yet
- Plinth Area Estmate For Laboratory, Test Range ,& ListDocument20 pagesPlinth Area Estmate For Laboratory, Test Range ,& ListShailendraNo ratings yet
- Certificate No. 273 Technical Methanol Grade A: No. Indicator Name Test Method Actual ValueDocument2 pagesCertificate No. 273 Technical Methanol Grade A: No. Indicator Name Test Method Actual Valueذياب الكايدNo ratings yet
- Word Sarcina TermicaDocument3 pagesWord Sarcina TermicaSergiu AlupoaeNo ratings yet
- CHM 213 - Exp 5Document9 pagesCHM 213 - Exp 5hafiqahNo ratings yet
- Pharmaceutics 13 00919 v2Document45 pagesPharmaceutics 13 00919 v24n4n4sNo ratings yet
- The Acid-Base Properties of WaterDocument3 pagesThe Acid-Base Properties of WaterRey DamnNo ratings yet
- Determination of Alcoholic Content of Asava and Arishta by Distillation Method and Specific Gravity MethodDocument4 pagesDetermination of Alcoholic Content of Asava and Arishta by Distillation Method and Specific Gravity MethodPrasanthi BodduNo ratings yet
- Exercise ProblemsDocument1 pageExercise ProblemsAshebirNo ratings yet
- ISO-2813-2014 Paints and Varnishes - DeterminationDocument11 pagesISO-2813-2014 Paints and Varnishes - DeterminationbonyjuniorNo ratings yet
- Sedat İnanDocument6 pagesSedat İnanRashid AhmedovNo ratings yet
- 01 Plant Design and Economics IntroductionDocument32 pages01 Plant Design and Economics Introductionهادی طاهریNo ratings yet
- 2020 Yearly Exam PaperDocument22 pages2020 Yearly Exam PaperYu-Tang LinNo ratings yet
- Calde™ Cast LW 146 CoDocument3 pagesCalde™ Cast LW 146 CoMohammed Ahtesham100% (1)
- Preparation and Assay of Acetyl PhosphateDocument4 pagesPreparation and Assay of Acetyl PhosphatecataawwwNo ratings yet
- Biospada Plus 60 Technical Data Sheet - CopiarDocument2 pagesBiospada Plus 60 Technical Data Sheet - CopiarPablo CuellarNo ratings yet
- Assignment 3 AdsorptionDocument12 pagesAssignment 3 Adsorptionshivam patelNo ratings yet
- Ceq Apsp eDocument27 pagesCeq Apsp eChess EnjoyerNo ratings yet
- Establishment of The ClausiusDocument7 pagesEstablishment of The ClausiusHoàng Ngọc HoànNo ratings yet
- Kepital F20 - 03Document2 pagesKepital F20 - 03Kumaar RanjanNo ratings yet
- FGM Mud Log Book Jan 2022Document61 pagesFGM Mud Log Book Jan 2022ongcchemist gd chitraNo ratings yet
- DLP ConcentrationDocument3 pagesDLP ConcentrationMae Amor EnorioNo ratings yet
- Food Chemistry: Ana Paula Craig, Adriana S. Franca, Leandro S. OliveiraDocument7 pagesFood Chemistry: Ana Paula Craig, Adriana S. Franca, Leandro S. OliveiraAlexánder Muñoz FerrerNo ratings yet
- Morrissey Lab Protocol For Preparing Phospholipid Vesicles by ExtrusionDocument3 pagesMorrissey Lab Protocol For Preparing Phospholipid Vesicles by ExtrusionVincent NguyenNo ratings yet