Professional Documents
Culture Documents
Boef Efo Jaef Ajfoef32
Uploaded by
Bush HsiehOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Boef Efo Jaef Ajfoef32
Uploaded by
Bush HsiehCopyright:
Available Formats
Review Article
Bacterial Infections of
Address correspondence to
Dr Karen L. Roos, Indiana
University Neuroscience
Center, 355 West 16th Street,
the Central Nervous Suite 4215, Indianapolis, IN
46202, kroos@iupui.edu.
Relationship Disclosure:
System Dr Roos has received personal
compensation as a senior
associate editor from MedLink
Neurology and for speaking
Karen L. Roos, MD, FAAN engagements from Emory
University, Marian University,
the University of California,
San Francisco, and the
ABSTRACT University of Virginia. Dr Roos
Purpose of Review: Bacterial infections of the central nervous system are neurologic receives royalties from
emergencies. Prompt recognition and treatment are essential not only to prevent Elsevier B.V. and Springer.
Unlabeled Use of
mortality, but also to decrease neurologic sequelae. This article focuses on the two Products/Investigational
most common central nervous system bacterial infections, bacterial meningitis and Use Disclosure:
spinal epidural abscess. Dr Roos reports no disclosure.
Recent Findings: Two outbreaks of serogroup B meningococcal disease have occurred * 2015, American Academy
of Neurology.
on US college campuses. The meningococcal vaccine given to young adults does not
contain serogroup B.
Summary: In bacterial meningitis and in bacterial spinal epidural abscess, the iden-
tification of and eradication of the pathogen with antimicrobial therapy is the easy part.
It is the recognition of the disorder, the understanding of which diagnostic studies to
obtain and their limitations, and the management of the neurologic complications that
require the expertise of a neurologist.
Continuum (Minneap Minn) 2015;21(6):1679–1691.
INTRODUCTION triad, primarily over the lack of, or in-
There are a number of bacterial infec- ability to detect, a stiff neck. This con-
tions of the central nervous system troversy was resolved by the recognition
(CNS), including meningitis, brain ab- that the majority of patients with bac-
scess, cranial and spinal epidural abscess, terial meningitis have fever (greater
subdural empyema, and suppurative than or equal to 38.5-C [101.3-F]) and
dural sinus thrombophlebitis, but this either headache, stiff neck, or an altered
article focuses on the two CNS bacterial level of consciousness.1Y3 Vomiting is
infections that neurologists encounter another symptom that is often present
the most often and in which they have but underrecognized as a symptom of
a critical role in diagnosis, manage- CNS infection.
ment, and lifetime care. Those infec- When bacterial meningitis is consid-
tions are bacterial meningitis and spinal ered a possibility, blood cultures should
epidural abscess. be obtained and empiric antimicrobial
and adjunctive therapy initiated. Em-
MENINGITIS piric antimicrobial therapy is based on
For two centuries, the clinical presenta- predisposing and associated conditions
tion of bacterial meningitis was rec- that predict the meningeal pathogen.
ognized as the classic triad of fever, Almost all recommended empiric an-
headache, and stiff neck. Then a number timicrobial regimens include a third- or
of years ago, articles began to appear fourth-generation cephalosporin plus
in the literature challenging the classic vancomycin. Ampicillin is added for
Continuum (Minneap Minn) 2015;21(6):1679–1691 www.ContinuumJournal.com 1679
Copyright © American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
Bacterial Infections
KEY POINTS
h The majority of patients coverage of Listeria monocytogenes Etiologic Organism
with bacterial meningitis where indicated (discussed later in this Despite the progress that has been made
have fever (greater than article), and metronidazole is added when with vaccination, the most common
or equal to 38.5-C the predisposing conditions of otitis, si- causative organisms of community-
[101.3-F]), and either nusitis, or mastoiditis are present.4 Prior acquired bacterial meningitis are Strep-
headache, stiff neck, or to or with the first dose of antibiotic tococcus pneumoniae and Neisseria
an altered level of therapy, dexamethasone (0.15 mg/kg meningitides. The tetravalent meningo-
consciousness. Vomiting every 6 hours for 2 to 4 days) is initiated coccal vaccine that is used to vaccinate
is another symptom that
in patients with suspected pneumococ- adolescents in the United States contains
is often present but
cal, meningococcal, or Haemophilus serogroups A, C, W-135, and Y. The vac-
underrecognized as a
symptom of central
influenzae type b meningitis.5Y7 Empiric cine does not contain serotype B, which
nervous system infection. therapy based on the predicted menin- is the causative organism of one-third
geal pathogen is listed in Table 7-1 and of cases of meningococcal disease in
h When bacterial meningitis
is considered a
the recommended doses of antibiotics industrialized countries.8 The pneumo-
possibility, blood cultures are listed in Table 7-2. After treatment coccal vaccines decrease the incidence
should be obtained and has been initiated, thoughtful considera- of acute otitis media and pneumonia,
empiric antimicrobial tion of etiologic organism, diagnostic but are not ‘‘meningitis vaccines.’’
and adjunctive studies, differential diagnosis, and treat- Predisposing and associated conditions
therapy initiated. ment can be undertaken. help predict the meningeal pathogen.
TABLE 7-1 Empiric Antimicrobial Therapy Based on the Predicted Meningeal Pathogen
Predisposing Condition Bacterial Pathogen Antibiotic
Children and adults; Streptococcus pneumoniae and Third- or fourth-generation
community acquired Neisseria meningitidis cephalosporin plus vancomycin
Otitis, mastoiditis, Streptococci species, gram-negative Third- or fourth-generation
sinusitis anaerobes (eg, Bacteroides species, cephalosporin plus vancomycin
Fusobacterium species), Staphylococcus plus metronidazole
aureus, Haemophilus species,
Enterobacteriaceae
Adults over the age S. pneumoniae, gram-negative Third- or fourth-generation
of 55 and people with bacilli, N. meningitidis, Listeria cephalosporin plus vancomycin
chronic illness monocytogenes, Haemophilus influenzae plus ampicillin
Endocarditis Viridans streptococci, S. aureus, Third- or fourth-generation
Streptococcus bovis, HACEK group, cephalosporin plus vancomycin
enterococci
Immunosuppressed S. pneumoniae, L. monocytogenes, H. Third- or fourth-generation
influenzae cephalosporin plus vancomycin
plus ampicillin
Postneurosurgical Staphylococci, gram-negative bacilli Vancomycin plus meropenem or
vancomycin plus ceftazidime
Intraventricular device Staphylococci, gram-negative Vancomycin plus meropenem or
bacilli, anaerobes vancomycin plus ceftazidime
plus metronidazole
HACEK = Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae.
B 2015 Karen L. Roos, MD, FAAN.
1680 www.ContinuumJournal.com December 2015
Copyright © American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
TABLE 7-2 Recommended Doses for the Antibiotics Commonly Used in the Treatment of
Bacterial Central Nervous System Infections
Antibiotic
Agent Total Daily Dosage, Route, and Dosing Intervals
Ampicillin Infants and children: 300 mg/kg/d IV in equally divided doses every 6 hours
Adults: 12 g/d IV in equally divided doses every 4Y6 hours
Cefepime Infants and children: 150 mg/kg/d IV in equally divided doses every 8 hours
Adults: 6 g/d IV in equally divided doses every 8 hours
Cefotaxime Infants and children: 225Y300 mg/kg/d IV in equally divided doses every 6Y8 hours
Adults: 8Y12 g/d IV in equally divided doses every 4Y6 hours
Ceftriaxone Infants and children: 80Y100 mg/kg/d IV in equally divided doses every 12 hours
Adults: 4 g/d IV in equally divided doses every 12 hours
Gentamicin Infants and children: 7.5 mg/kg/d IV in equally divided doses every 8 hours
Adults: 5 mg/kg/d IV in equally divided doses every 8 hours
Meropenem Infants and children: 120 mg/kg/d IV in equally divided doses every 8 hours
Adults: 6 g/d IV in equally divided doses every 8 hours
Metronidazole Infants and children: 30 mg/kg/d IV in equally divided doses every 6 hours
Adults: Loading dose of 15 mg/kg IV over 1 hour followed by 7.5 mg/kg IV every
6 hours (maximum daily dose is 4 g/d)
Nafcillin Infants and children: 100Y200 mg/kg/d IV in equally divided doses every 6 hours
Adults: 9Y12 g/d IV in equally divided doses every 4 hours
Penicillin G Infants and children: 0.3 million units/kg/d IV in equally divided doses every 4Y6 hours
Adults: 12Y24 million units/d IV in equally divided doses every 4Y6 hours
Rifampin Infants and children: 10Y20 mg/kg/d IV in equally divided doses every 12Y24 hours
Adults: 600Y1200 mg/d IV in equally divided doses every 12 hours
Vancomycina Infants and children: 40Y60 mg/kg/d IV in equally divided doses every 6 hours
Adults: 45Y60 mg/kg/d IV in equally divided doses every 8Y12 hours
Chemoprophylaxis Infants and children: Rifampin 10Y20 mg/kg/d for 2 days
Neisseria meningitidis
Adults: Rifampin 600 mg orally or IV 2 times/d for 2 days or ceftriaxone 250 mg IM once daily
for 1 day
IV = intravenous.
a
Intraventricular vancomycin administration: children 10 mg/d, adults 20 mg/d.
B 2015 Karen L. Roos, MD, FAAN.
In the patient with pneumonia, S. (including S. pneumoniae), gram-
pneumoniae is a likely meningeal path- negative anaerobes, Staphylococcus
ogen. When meningitis is suspected in a aureus, Haemophilus species, or En-
patient with a recent history of otitis, terobacteriaceae. The most common
sinusitis, or mastoiditis, the etiologic causative organisms of bacterial men-
organisms are streptococci species. ingitis in the patient who has had a
Continuum (Minneap Minn) 2015;21(6):1679–1691 www.ContinuumJournal.com 1681
Copyright © American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
Bacterial Infections
KEY POINTS
h The most common neurosurgical procedure are staphylo- count and a CT scan has been per-
causative organisms of cocci, gram-negative bacilli, and anaer- formed. Blood cultures, a C-reactive pro-
community-acquired obes. It is important to recognize when tein, and a serum procalcitonin should
bacterial meningitis are an anaerobe may be the meningeal path- be obtained. A normal C-reactive pro-
Streptococcus ogen as that possibility necessitates the tein has a high negative predictive value
pneumoniae and addition of metronidazole to the em- in the diagnosis of bacterial meningi-
Neisseria meningitides. piric regimen (Case 7-1). tis.5 An elevated serum procalcitonin
h The tetravalent Include L. monocytogenes as a pos- concentration occurs in severe bacterial
meningococcal vaccine sible meningeal pathogen in patients infections. The serum procalcitonin is
does not contain who are pregnant, adults over the age an additional helpful test in differen-
serogroup B. Even if of 55, and individuals with impaired tiating bacterial meningitis from viral
individuals have been
cell-mediated immunity due to chronic meningitis when the CSF Gram’s stain
vaccinated with the
meningococcal vaccine,
illness, organ transplantation, acquired is negative.
they are still at risk for immunodeficiency syndrome (AIDS), The gold standard for the diagnosis
meningitis due to malignancy, or immunosuppressive ther- of bacterial meningitis is, of course,
serogroup B meningococci. apy. This is important because these analysis of the CSF. The neurologist is
h A normal C-reactive predisposing factors necessitate the ad- critical to the care of the patient with
protein has a high dition of ampicillin to the empiric anti- meningitis because few physicians other
negative predictive microbial regimen. In general, avoid the than neurologists have the skills to
value in the diagnosis use of gentamicin whenever possible, perform lumbar punctures; even fewer
of bacterial meningitis. but gentamicin is added to the empiric can manipulate a manometer to obtain
antimicrobial regimen in critically ill an opening pressure or have the knowl-
patients suspected of having L. mono- edge to send the critical tests on CSF. In
cytogenes meningitis. addition, the eradication of the menin-
geal pathogen is the easy part; it is the
Diagnostic Studies recognition and management of the
By the time the neurologist is consulted neurologic complications that is much
by the emergency department, patients more difficult, which are discussed later
have had a peripheral white blood cell in this article.
Case 7-1
A 19-year-old college freshman presented to the emergency department
reporting chills, headache, and vomiting for the last 12 hours. He had
recently been treated with amoxicillin for otitis media. On examination, his
temperature was 38.7-C (101.7-F), and he had photophobia and was
increasingly lethargic. The neurologist obtained blood cultures and treated
him with dexamethasone 0.15 mg/kg, ceftriaxone 2 g, vancomycin 15 mg/kg,
metronidazole 500 mg, and acyclovir 10 mg/kg. Spinal fluid analysis
demonstrated an opening pressure of 480 mm water; 868 white blood
cells/mm3, with a predominance of polymorphonuclear leukocytes; a glucose
concentration of 6 mg/dL; and a protein concentration of 480 mg/dL. CSF
Gram’s stain demonstrated a gram-negative organism, and the culture grew
Fusobacterium necrophorum.
Comment. Empiric therapy of bacterial meningitis includes a third- or
fourth-generation cephalosporin plus vancomycin. The predisposing factor of
otitis media put this patient at risk for meningitis due to a gram-negative anaerobe;
thus, the addition of metronidazole to the empiric regimen is indicated.
1682 www.ContinuumJournal.com December 2015
Copyright © American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
KEY POINT
Table 7-3 lists diagnostic tests to manometer, as opening pressures h CSF should be handled
obtain on CSF. The classic CSF abnor- as high as or higher than 500 mm with care and examined
malities in bacterial meningitis include water are not unusual. promptly for white
the following: & A pleocytosis of polymorphonuclear blood cells after it
& Increased opening pressure. It is leukocytes. CSF should be is obtained.
always a good idea to put the handled with care and examined
additional extension piece on the promptly for white blood cells
TABLE 7-3 Cerebrospinal Fluid Diagnostic Studies for Meningitis
b Cell Count With Differential
b Glucose and Protein Concentration
b Stain and Culture
Gram’s stain and bacterial culture
India ink and fungal culture
Viral culture
Acid fast smear and Mycobacterium tuberculosis culture
b Antigens/Antibodies if Fungal Meningitis Is in the Differential
Cryptococcal polysaccharide antigen
Histoplasma polysaccharide antigen
Coccidioides immitis complement fixation antibody
b Polymerase Chain Reaction (PCR)
Broad range bacterial (16S ribosomal DNA)
Specific meningeal pathogen
Reverse transcriptase for enteroviruses
Herpes simplex virus type 1 and type 2
West Nile virus
Epstein-Barr virus
Varicella-zoster virus
NAAT for M. tuberculosis
Human immunodeficiency virus (HIV) RNA
b Antibodies
Herpes simplex virus (serum and CSF IgG to calculate antibody ratio)
Varicella-zoster virus IgM and IgG antibody index
Arthropod-borne viruses (West Nile virus IgM)
Borrelia burgdorferi antibody index
CSF = cerebrospinal fluid; DNA = deoxyribonucleic acid; IgG = immunoglobulin G; IgM =
immunoglobulin M; NAAT = nucleic acid amplification test; RNA = ribonucleic acid.
B 2015 Karen L. Roos, MD, FAAN.
Continuum (Minneap Minn) 2015;21(6):1679–1691 www.ContinuumJournal.com 1683
Copyright © American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
Bacterial Infections
KEY POINTS
h In general, a decreased after it is obtained. If the & On the initial CSF analysis, in addition
CSF glucose CSF is allowed to sit around to the presence or absence of a
concentration is evidence for hours before it is examined, pleocytosis, the identification of the
of both a potentially allowed to get warm, or predominant cell type being either
treatable and potentially launched through a hospital polymorphonuclear leukocytes or
fatal central nervous tube system, the white lymphocytes, the presence or
system infection. blood cell count will be absence of a decreased glucose
h The reverse transcriptase erroneously low. concentration, and the results of the
polymerase chain Gram’s stain, three polymerase
reaction (RT-PCR) for & A glucose concentration of less
chain reaction (PCR) assays are
enteroviruses is reported than 40 mg/dL. It is not unusual
important in the initial diagnostic
in 4 hours. As to have a CSF glucose concentration
stage. These are the 16S ribosomal
enteroviruses are the in the single digits. Always obtain a
RNA (rRNA) conserved sequence
most common cause of serum glucose concentration
viral meningitis, a broad-based bacterial PCR, the
immediately before performing a
positive CSF RT-PCR reverse transcriptase PCR
lumbar puncture when bacterial
for enteroviruses is (RT-PCR) for enteroviruses, and
meningitis is a possibility as
very helpful in the herpes simplex virus type 1
hyperglycemia increases the CSF
management decisions. (HSV-1) and the herpes simplex
glucose concentration. A normal
virus type 2 (HSV-2) PCRs. The
CSF-to-serum ratio is 0.6. A
results for the 16S rRNA bacterial
CSF-to-serum ratio of less than
PCR take 48 hours or longer to
0.40 is highly predictive of bacterial
be reported, and typically by the
meningitis. In general, a decreased
time this result is available, the
CSF glucose concentration is
organism has been definitively
evidence of both a potentially
identified by CSF culture. This
treatable and potentially fatal
PCR is expected to be most helpful
CNS infection (ie, bacterial,
in those patients with a negative
fungal, tuberculous, or
Gram’s stain and culture as the
carcinomatous meningitis).
result of pretreatment with antibiotics
& An elevated protein concentration. for their predisposing condition
Any process that increases the (eg, pneumonia, otitis, or sinusitis).
permeability of the blood-brain Fortunately, the RT-PCR for
barrier increases the CSF enteroviruses is reported in
protein concentration. 4 hours. As enteroviruses are the
& Gram’s stain is positive in most common cause of viral
identifying the organism in 60% meningitis, a positive CSF RT-PCR
to 90% of cases of bacterial for enteroviruses is very helpful in
meningitis, but the probability management decisions.
of a positive Gram’s stain is & The CSF lactate concentration is,
dependent on the number of in general, nonspecific and only
organisms present. The results recommended in the diagnosis of
of a Gram’s stain are usually bacterial meningitis in the
available in 4 hours. postoperative neurosurgical patient.
& The CSF culture result is not A CSF lactate concentration of
only critical for the identification 4.0 mmol/L or more is positive.
of the meningeal pathogen, & The latex agglutination test was
but also for antimicrobial a great test that is no longer
sensitivity testing. routinely available.
1684 www.ContinuumJournal.com December 2015
Copyright © American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
KEY POINTS
Differential Diagnosis CT or MRI. CSF Gram’s stain and culture h Patients with viral
In the patient with a clinical presenta- are negative, and the symptoms resolve meningitis do not have
tion of fever, headache, and stiff neck, promptly when the offending medica- an altered level of
the leading disease in the differential tion is discontinued.9 consciousness, seizures, or
diagnosis is viral meningitis. Patients Herpes simplex virus encephalitis is focal neurologic deficits.
with viral meningitis are awake and in the differential diagnosis in any pa- h Medication-induced
alert. They are often sitting on the ex- tient with fever and headache. In the meningitis may present
amination table reporting about the majority of patients with herpes simplex with fever, headache,
severity of their headache. Patients with virus encephalitis, fluid-attenuated in- stiff neck, and an
viral meningitis do not have an altered version recovery (FLAIR) and diffusion- altered level
level of consciousness, seizures, or focal weighted MRI sequences will show of consciousness.
neurologic deficits. Patients with bacte- hyperintensity in the inferior and medial h If the initial CSF
rial meningitis have a progressive de- temporal lobes within 48 hours of symp- polymerase chain
crease in the level of consciousness from tom onset. If the initial CSF PCR for reaction (PCR) for
lethargy to stupor to obtundation to HSV-1 is negative and the suspicion herpes simplex virus
coma. They are not sitting up, but rather, for herpes simplex virus encephalitis is type 1 is negative and the
lying down. Tuberculous meningitis strong, repeat the CSF analysis. A false- suspicion for herpes
negative PCR for HSV-1 may occur in simplex virus encephalitis
may also present with the apoplectic
is strong, repeat the
onset of fever and headache, and thus the first 72 hours of symptom onset.
spinal fluid analysis. A
the recommendation that CSF analy- An infectious intracranial mass lesion
false-negative PCR for
sis include a PCR for Mycobacterium will be readily visualized on neuroimag- herpes simplex virus
tuberculosis, an acid-fast smear, and a ing. A subarachnoid hemorrhage may type 1 may occur in the
M. tuberculosis culture. A common term have a similar presentation to bacterial first 72 hours of
used instead of PCR, especially for meningitis, with the sudden onset of symptom onset.
M. tuberculosis, is nucleic acid ampli- headache and stiff neck. Neuroimaging h The acute complications
fication test (NAAT). Fungal meningitis and spinal fluid analysis will demon- of bacterial meningitis
tends to have a more insidious onset strate hemorrhage, prompting CT angi- are increased intracranial
over several weeks, with headache, fever, ography for an intracranial aneurysm. pressure, cerebral
cranial nerve palsies, and an altered edema, coma, ischemic
level of consciousness. Management and hemorrhagic
The list of medications that can cause The management of bacterial meningi- cerebrovascular
drug-induced meningitis is ever increas- disease, venous sinus
tis includes the eradication of the menin-
ing owing to the increasing number of thrombosis, cranial
geal pathogen with antimicrobial therapy nerve palsies, and
immunomodulating agents. Some of the
as well as the prevention, as much as vestibulocochlear
better-known culprits for drug-induced
possible, and treatment of the acute com- involvement.
meningitis are penicillins, cephalospo-
rins, sulfonamides, trimethoprim, IV im- plications of this infection. The acute
munoglobulin (IVIg), azathioprine, complications of bacterial meningitis
cytosine arabinoside, and nonsteroidal are increased intracranial pressure, cere-
anti-inflammatory medications. The clin- bral edema, coma, ischemic and hemor-
ical presentation includes fever, head- rhagic cerebrovascular disease, venous
ache, stiff neck, lethargy, confusion, sinus thrombosis, cranial nerve palsies,
seizures, and coma. CSF analysis may and vestibulocochlear involvement.
demonstrate an increased opening Empiric antimicrobial therapy is mod-
pressure, a pleocytosis of polymorpho- ified once the meningeal pathogen has
nuclear leukocytes, and a decreased been identified and the results of anti-
glucose concentration. There may be microbial sensitivity testing are available.
diffuse enhancement of the meninges Unfortunately, it is both the multiplica-
after the administration of contrast on tion of the bacterial organisms and the
Continuum (Minneap Minn) 2015;21(6):1679–1691 www.ContinuumJournal.com 1685
Copyright © American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
Bacterial Infections
lysis of bacteria by bactericidal antibi- tive prospective double-blind placebo-
otics with the release of bacterial cell controlled trials in 200 infants and
wall components that initiates the cas- children and demonstrated a significantly
cade that leads to the neurologic com- reduced frequency of moderate, severe,
plications. The release of bacterial cell or profound bilateral sensorineural hear-
wall components leads to the produc- ing loss in the patients who received
tion of inflammatory cytokines and che- dexamethasone. Odio and colleagues14
mokines by monocytes, macrophages, conducted a placebo-controlled double-
brain astrocytes, and microglial cells blind trial of dexamethasone therapy
(CNS-macrophage-equivalent cells). in 101 infants and children and demon-
The inflammatory cytokines are the en- strated that neurologic sequelae oc-
dogenous host mediators of inflamma- curred significantly more often in the
tion and are directly responsible for patients who were given placebo. These
altered blood-brain barrier permeability, studies led the American Academy of
decreased cerebral blood flow, the Pediatrics to recommend that the use
purulent exudate in the subarachnoid of dexamethasone be considered in
space, and the production of excitatory infants and children 2 months of age
amino acids and reactive oxygen and and older with proven or suspected
nitrogen species. The neurologic com- bacterial meningitis and that the initial
plications that occur as a result of this dose of dexamethasone be given with
are vasogenic, interstitial, and cytotoxic the initial dose of antimicrobial therapy.15
edema; ischemic and hemorrhagic stroke; The initial reluctance to use dexa-
venous sinus thrombosis; cranial nerve methasone in all patients was because
palsies; seizures; increased intracranial in the landmark studies, the majority
pressure; and coma. of patients were infants and children,
Armed with this understanding, the and they had meningitis due to H.
obvious intervention to prevent the influenzae type b. The efficacy of dexa-
pathophysiologic consequences and re- methasone had yet to be proven in adults
sultant neurologic complications from or in meningitis due to S. pneumoniae
inflammatory cytokines is to prevent the or N. meningitidis. The experimental
production of the inflammatory cyto- models of meningitis suggested that
kines. Thus, for decades, interest in dexamethasone should be efficacious
dexamethasone therapy has grown. in meningitis caused by either a gram-
Using experimental models of menin- positive or a gram-negative organism as
gitis,10Y12 investigators demonstrated each had a component (or components)
that dexamethasone inhibited the syn- of their cell wall that stimulated the pro-
thesis of the inflammatory cytokines, duction of the inflammatory cytokines.
reduced meningeal inflammation, de- In 2002, de Gans and colleagues16
creased cerebral edema and intracranial published the results of their prospec-
pressure, and stabilized the blood-brain tive, randomized, double-blind mul-
barrier (decreasing vasogenic edema, ticenter trial of dexamethasone in
leakage of serum proteins, and forma- 301 adults with acute bacterial menin-
tion of the purulent exudate in the sub- gitis. S. pneumoniae was the causative
arachnoid space). The original clinical organism in 58 of the patients in the
trials on dexamethasone in bacterial dexamethasone group and 50 in the
meningitis were done by Lebel and col- placebo group. Of the patients with
leagues13 in Dallas and Odio and col- pneumococcal meningitis in the dexa-
leagues14 in Costa Rica. Lebel and methasone group, 26% had an unfavor-
colleagues13 conducted two consecu- able outcome, compared with 52% in
1686 www.ContinuumJournal.com December 2015
Copyright © American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
KEY POINTS
the placebo group. Of the patients with with antibiotic therapy and is getting h The Infectious Diseases
pneumococcal meningitis who received progressively worse. The answer is the Society of America
dexamethasone, 14% died, compared evidence supports the use of dexameth- Practice Guidelines
to 34% of those who received placebo. asone at the initiation of therapy and for acknowledge that
After the initiation of nationwide dexa- the first 4 days. After the inflammatory ‘‘some authorities
methasone therapy in adults with pneu- cytokines have initiated the cascade, would initiate
mococcal meningitis in the Netherlands, therapy should be directed at the spe- dexamethasone in all
there was a decline in the fatality rate cific management of complications adults [with suspected
from 30% to 20%.17 such as increased intracranial pressure, bacterial meningitis],
Both the Infectious Diseases Society seizures, or stroke. because the etiology of
meningitis is not always
of America Practice Guidelines for the Cerebral edema with increased intra-
ascertained at
Management of Bacterial Meningitis5 cranial pressure in bacterial meningitis initial evaluation.’’
and the European Federation of Neu- is managed as it is when it occurs as a
rological Societies Guideline on the complication of other CNS disorders, h The evidence supports the
use of dexamethasone
Management of Community-Acquired with elevation of the head of the bed,
in the treatment of
Bacterial Meningitis6 recommend the hyperventilation, and mannitol. Seizures bacterial meningitis at
use of dexamethasone in adults with are often the result of cerebrovascular the initiation of therapy
suspected or proven pneumococcal complications and are treated accord- and for the first 4 days of
meningitis. The Infectious Diseases ingly. Patients who develop neurologic antimicrobial therapy.
Society of America Practice Guidelines complications during the acute phase
also acknowledge that ‘‘some au- of bacterial meningitis are at risk for
thorities would initiate dexamethasone permanent neurologic sequelae, includ-
in all adults [with suspected bacterial ing cognitive impairment, hearing loss,
meningitis], because the etiology of and seizure disorders.
meningitis is not always ascertained
at initial evaluation.’’5 In fact, a 2012 SPINAL EPIDURAL ABSCESS
study demonstrated a favorable trend The incidence of spinal epidural ab-
toward reduced rates of death and scess has increased for two reasons:
hearing loss and no evidence that (1) Infections due to S. aureus, the most
dexamethasone was harmful in pa- common causative organism, have in-
tients with meningococcal meningitis.7 creased, and (2) diabetes mellitus, an
The European Federation of Neuro- increasingly common condition, is the
logical Societies Task Force6 also rec- single most important risk factor for
ommends that dexamethasone be
the development of a spinal epidural
administered to children with suspected
abscess. Neurologists are often con-
pneumococcal or H. influenzae
sulted for ‘‘weakness,’’ making the timely
type b meningitis.
recognition and diagnosis of a spinal
The American Academy of Pediat-
epidural abscess an entity that ‘‘we
rics, the Infectious Diseases Society of
own.’’ Neurologists also care for these
America, and the European Federation
patients long term and, as such, have the
of Neurological Societies all agree that
best understanding of the prognosis.
the dose of dexamethasone is 0.15 mg/kg
A spinal epidural abscess has a classic
every 6 hours for 2 to 4 days and that
the initial dose of dexamethasone should presentation as described by Heusner
be given either before or with the initial in 1948.18
dose of antimicrobial therapy. & Back pain at the level of
Neurologists are often asked if dexa- infection. In addition, fever
methasone should be started 4 days or may be present, but is not
more after the patient has been treated universally present.
Continuum (Minneap Minn) 2015;21(6):1679–1691 www.ContinuumJournal.com 1687
Copyright © American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
Bacterial Infections
KEY POINTS
h Staphylococcus aureus & Pain in a radicular distribution upper extremity weakness. The findings
is the most common in the extremities, or an on neurologic examination are the
causative organism intercostal thoracic same as those with a spinal epidural
of a spinal epidural dermatomal distribution. abscess and include appendicular
abscess, followed by & Paresis, loss of sensation below the weakness, a well-defined sensory level,
gram-negative bacilli. level of the lesion, and loss of hypotonia, and (acutely) absent or de-
h When spinal epidural bowel and bladder control. creased muscle stretch reflexes. Guillain-
abscess is suspected, & Paralysis. Barré syndrome presents as an ascending
the level of the lesion weakness with loss of the muscle stretch
must be determined Etiologic Organism reflexes. Urinary retention may develop
by the neurologic in the course of Guillain-Barré syndrome
A spinal epidural abscess develops by
examination to narrow as a symptom of autonomic dysfunc-
one of the following mechanisms: (1) as
(but not limit) the
a result of hematogenous dissemina- tion, but is not a typical feature of the
extent of imaging to
tion from a remote site of infection dur- acute presentation.
be obtained.
ing the course of bacteremia; (2) from a Non-neurologists often want to im-
contiguous site of infection (eg, verte- age the entire neuraxis in a patient with
bral osteomyelitis/discitis, decubitus weakness, but the length of time it takes
ulcers, infected abdominal wounds, soft to perform such an extensive MRI delays
tissue paravertebral abscesses); (3) as a obtaining the MRI. It is critical to local-
result of trauma, or from direct inocu- ize the lesion by the findings on the
lation during spinal instrumentation or neurologic examination and focus the
epidural analgesia. S. aureus is the most imaging on the predicted area of the spi-
common causative organism, followed by nal epidural abscess as well as a few
gram-negative bacilli. Empiric antibiotic levels above and below that (Case 7-2).
therapy should include a combination
Diagnostic Studies
of vancomycin and a third- or fourth-
generation cephalosporin. Routine studies for suspected bacterial
infection of the CNS include complete
Differential Diagnosis blood count with differential, sedimen-
The differential diagnosis is defined tation rate, C-reactive protein, serum
by the neurologic examination, specifi- procalcitonin, and blood cultures. It is
cally the extent and tempo of the devel- extremely difficult to obtain an urgent
opment of appendicular weakness, the MRI of the entire neuraxis. The level
presence or absence of a sensory level, of the lesion must be determined by
and the presence or absence of bowel the neurologic examination to narrow
and bladder involvement. Fever and (but not limit) the extent of imaging to
back pain without signs of spinal root be obtained.
or cord compression may be due to
osteomyelitis and discitis. The two most Management
common diseases in the differential Patients with spinal epidural abscess
diagnosis of spinal epidural abscess are nearly always go to surgery; the pro-
transverse myelitis and Guillain-Barré cedure that is done is a decompres-
syndrome. The clinical presentation of sive laminectomy at one or more levels
transverse myelitis is that of back pain with drainage of the abscess. Gram’s
followed by lower extremity weakness stain and culture of the purulent ma-
and bowel and bladder dysfunction. terial obtained at the time of surgery
Transverse myelitis affecting the cervi- allow for identification of the infect-
cal cord presents with neck pain and ing organism and modification of the
1688 www.ContinuumJournal.com December 2015
Copyright © American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
Case 7-2
A 52-year-old man was admitted to the hospital reporting abdominal pain, slowly progressive weakness
in his extremities associated with neck pain, and dysphagia. Beginning 2 weeks prior to hospitalization, he
noted difficulty walking, followed by difficulty with the strength in his left arm. At the time of admission,
he was catheterized, and 2 liters of urine were removed. He had had an indwelling catheter since
admission. His admitting physician attributed his weakness to his poor oral intake and deconditioning.
He was evaluated by a gastroenterologist, who performed esophagogastroduodenoscopy, which was
normal. An otolaryngologist also evaluated the patient and found no abnormalities in his throat. On
hospital day 3, he developed a fever, and a complete blood count demonstrated a neutrophilic pleocytosis.
He was treated for an Escherichia coli urinary tract infection. Neurology was consulted when he
developed weakness in his right arm. On neurologic examination, he had bilateral lower extremity
plegia, asymmetric upper extremity hypotonic paresis, and a C2 sensory level. An emergent MRI of the
cervical spine was obtained (Figure 7-1), which showed a C3 epidural abscess due to a paravertebral abscess.
He was taken to the operating room for emergent
decompression and drainage of the abscess.
Gram’s stain of the purulent material obtained
from C2 revealed gram-negative bacilli, and the
culture grew E. coli. The neurosurgeon noted no
pulsation of the cord after decompression and
remarked about the presence of thrombosed
epidural veins. The patient did not recover
strength in any of his extremities.
Comment. The patient’s report of dysphagia
was the focus of evaluation during the first few
days of his hospitalization. It was not until he
developed worsening weakness under
observation in the hospital that neurology was
consulted. Despite emergent decompressive
surgery, he did not regain function in any of his
appendicular musculature. A critical piece to
being able to offer an opinion on the prognosis
is what the neurosurgeon sees with his or her
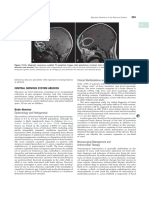
eyes on the operative field. This patient had FIGURE 7-1 Imaging of the patient in Case 7-2.
MRI of C3 spinal epidural abscess
infarction of the spinal cord due to septic (arrow) due to a paravertebral abscess.
thrombophlebitis and was not likely to recover
a significant degree of function.
antimicrobial coverage. Antimicrobial ative note, he or she should be asked KEY POINT
sensitivity testing of the bacterial culture to do so. An understanding of the path- h An understanding of the
ophysiology of the spinal cord injury pathophysiology of the
is critical to ensure that the organism
spinal cord injury from
is sensitive to the antibiotic. from the epidural abscess is critical to
an epidural abscess is
predicting the extent of recovery, to
critical to predicting the
Prognosis the degree that it can be predicted. extent of recovery, to
To address the prognosis, the neuro- The neurologic deficits from a spinal the degree that it can
surgeon should answer two questions: epidural abscess are due to one of or a be predicted.
(1) Did the spinal cord pulsate after combination of the following: (1) com-
decompression? and (2) Was there evi- pression of the spinal cord, (2) com-
dence of thrombosed arteries or veins? pression of the arterial blood supply,
If the neurosurgeon does not address (3) arterial or venous thrombosis, or
the likely pathophysiology in the oper- (4) septic thrombophlebitis. When the
Continuum (Minneap Minn) 2015;21(6):1679–1691 www.ContinuumJournal.com 1689
Copyright © American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
Bacterial Infections
KEY POINTS
h When compression is pathophysiology of the neurologic def- This is a devastating disease, and now
the mechanism of injury icits is due to spinal cord compression, that the incidence of this disease is in-
in spinal epidural ‘‘The single most important predictor creasing, it is important to focus efforts
abscess, improvement of the final neurologic outcome is the on both prevention and treatment. Be-
from decompressive patient’s neurologic status immediately cause of the common symptom of back
surgery can be expected. before surgery.’’19 Patients who undergo pain, diagnosis of spinal epidural abscess
When vascular pathology surgery with back pain or back pain and is often delayed. The presence of fever
is the mechanism of radicular pain are expected to have a and focal back pain and tenderness war-
spinal cord injury, good outcome with no neurologic de- rants imaging of the symptomatic level
recovery postsurgery ficits. Those who undergo surgery of the spine. In the patient with a pre-
is not likely.
with paresis are expected to have disposing condition, such as diabetes
h The presence of fever either no weakness or a lesser degree mellitus or S. aureus bacteremia, and,
and focal back pain and of weakness postoperatively. Patients thus, an increased risk for spinal epi-
tenderness warrants who are plegic but operated on within dural abscess, a concern of back pain
imaging of the
24 to 36 hours of the development of should be handled with the same sense
symptomatic level of
paralysis are expected to regain some of urgency as a report of chest pain.
the spine.
strength in the paralyzed extremities.19
Understanding of compression of CONCLUSION
the spinal cord as the mechanism of Bacterial meningitis and spinal epidural
injury in spinal epidural abscess came abscess are neurologic emergencies that
from experimental models. S. aureus require the expertise of a neurologist.
was directly inoculated into the epidu- Although an infectious disease physi-
ral space of rabbits to promote abscess cian can contribute to antimicrobial
formation. In this model, spinal cord selection and a neurosurgeon can con-
compression from epidural abscess for- tribute to decompressing a mass lesion,
mation at the site of inoculation re- neurologists understand the manage-
sulted in paralysis.20 When compression ment of these infections and their de-
is the mechanism of injury, improve- vastating consequences.
ment from decompressive surgery can
REFERENCES
be expected.
1. Durand ML, Calderwood SB, Weber DJ,
When vascular pathology is the me- et al. Acute bacterial meningitis in adults.
chanism of spinal cord injury in spinal A review of 493 episodes. N Engl J Med
1993;328(1):21Y28.
epidural abscess, recovery postsurgery
is not likely. The vascular pathology may 2. van de Beek D, de Gans J, Spanjaard L, et al.
Clinical features and prognostic factors in
be an arteritis with spinal cord ischemia, adults with bacterial meningitis. N Engl
an arterial or venous thrombosis with J Med 2004;351(18):1849Y1859. doi:10.1056/
NEJMoa040845.
spinal cord ischemia, or a thrombophle-
bitis with hypoxia secondary to ob- 3. Thomas KE, Hasbun R, Jekel J, Quagliarello VJ.
The diagnostic accuracy of Kernig’s sign,
structed venous drainage of the cord.21 Brudzinski’s sign, and nuchal rigidity in adults
Thrombophlebitis of the leptomenin- with suspected meningitis. Clin Infect Dis
geal vessels and arterial occlusion have 2002;35(1):46Y52. doi:10.1086/340979.
been visualized intraoperatively and at 4. Roos KL, van de Beek D. Bacterial
meningitis. In: Roos KR, Tunkel AR, eds.
autopsy. In these reports, cord com- Handbook of clinical neurology. Edinburgh:
pression was not the mechanism of Elsevier, 2010:51Y63.
injury.22,23 It is also possible that both 5. Tunkel AR, Hartman BJ, Kaplan SL,
cord compression and vascular pathol- et al. Practice guidelines for the
management of bacterial meningitis.
ogy are the mechanism of spinal cord Clin Infect Dis 2004;39(9):1267Y1284.
injury in any individual patient. doi:10.1086/425368.
1690 www.ContinuumJournal.com December 2015
Copyright © American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
6. Chaudhuri A, Martinez-Martin P, 14. Odio CM, Faingezicht I, Paris M, et al. The
Kennedy PG, et al. EFNS guideline on the beneficial effects of early dexamethasone
management of community-acquired administration in infants and children with
bacterial meningitis: report of an EFNS bacterial meningitis. N Engl J Med
Task Force on acute bacterial meningitis in 1991;324(22):1525Y1531. doi:10.1056/
older children and adults. Eur J Neurol NEJM199105303242201.
2008;15(7):649Y659. doi:10.1111/j.1468-1331.
15. American Academy of Pediatrics Committee
2008.02193.x.
on Infectious Diseases: dexamethasone
7. Heckenberg SG, Brouwer MC, van der Ende A, therapy for bacterial meningitis in infants
van de Beek D. Adjunctive dexamethasone and children. Pediatrics 1990;86(1):130Y133.
in adults with meningococcal meningitis.
16. de Gans J, van de Beek D; European
Neurology 2012;79(15):1563Y1569.
Dexamethasone in Adulthood Bacterial
doi:10.1212/WNL.0b013e31826e2684.
Meningitis Study Investigators. Dexamethasone
8. Tan LK, Carlone GM, Borrow R. Advances in adults with bacterial meningitis. N Engl J
in the development of vaccines against Med 2002;347(20):1549Y1556. doi:10.1056/
Neisseria meningitidis. N Engl J Med 2010; NEJMoa021334.
362(16):1511Y1520. doi:10.1056/
17. Brouwer MC, Heckenberg SG, de Gans J, et al.
NEJMra0906357.
Nationwide implementation of adjunctive
9. De Marcaida JA, Reik L. Disorders that mimic dexamethasone therapy for pneumococcal
central nervous system infections. Neurol meningitis. Neurology 2010;75(17):1533Y1539.
Clin 1999;17(4):901Y941. doi:10.1212/WNL.0b013e3181f96297.
10. Mustafa MM, Ramilo O, Olsen KD, et al. 18. Heusner AP. Nontuberculous spinal epidural
Tumor necrosis factor in mediating abscess. N Engl J Med 1948;239(23):845Y854.
experimental Haemophilus influenzae type B
19. Darouiche RO. Spinal epidural abscess.
meningitis. J Clin Invest 1989;84(4):
N Engl J Med 2006;355(19):2012Y2020.
1253Y1259. doi:10.1172/JCI114292.
doi:10.1056/NEJM197509042931001.
11. Kern JA, Lamb RJ, Reed JC, et al.
20. Feldenzer JA, McKeever PE, Schaberg DR,
Dexamethasone inhibition of interleukin
et al. Experimental spinal epidural abscess:
1 beta production by human monocytes.
a pathophysiological model in the rabbit.
Posttranscriptional mechanisms. J Clin
Neurosurgery 1987;20(6):859Y867.
Invest 1988;81(1):237Y244. doi:10.1172/
JCI113301. 21. Akalan N, Ozgen T. Infection as a cause of
spinal cord compression: a review of 36 spinal
12. Tauber MG, Khayam-Bashi H, Sande MA.
epidural abscess cases. Acta Neurochir (Wien)
Effects of ampicillin and corticosteroids on
2000;142(1):17Y23. doi:10.1007/s007010050002.
brain water content, cerebrospinal fluid
pressure, and cerebrospinal fluid lactate 22. van de Warrenburg BP, Wesseling P,
levels in experimental pneumococcal Leyten QH, Boerman RH. Myelopathy due
meningitis. J Infect Dis 1985;151(3):528Y534. to spinal epidural abscess without cord
doi:10.1093/infdis/151.3.528. compression: a diagnostic pitfall. Clin
Neuropathol 2004;23(3):102Y106.
13. Lebel MH, Freij BJ, Syrogiannopoulos GA,
et al. Dexamethasone therapy for bacterial 23. Iwasaki M, Yano S, Aoyama T, et al. Acute
meningitis. Results of two double-blind, onset intramedullary spinal cord abscess
placebo-controlled trials. N Engl J Med 1988; with spinal artery occlusion: a case report
319(15):964Y971. doi:10.1056/ and review. Eur Spine J 2011;20(suppl 2):
NEJM198810133191502. S294YS301. doi:10.1007/s00586-011-1703-z.
Continuum (Minneap Minn) 2015;21(6):1679–1691 www.ContinuumJournal.com 1691
Copyright © American Academy of Neurology. Unauthorized reproduction of this article is prohibited.
You might also like
- Microbiology - A Human Perspective (PDFDrive)Document865 pagesMicrobiology - A Human Perspective (PDFDrive)Lê Hạ Long Hải100% (3)
- Covid Vaccines from a Spiritual Perspective: Consequences for the Soul and Spirit and for Life after DeathFrom EverandCovid Vaccines from a Spiritual Perspective: Consequences for the Soul and Spirit and for Life after DeathRating: 5 out of 5 stars5/5 (1)
- Dr. Klinghardt Lyme-Co-infections-ProtocolDocument4 pagesDr. Klinghardt Lyme-Co-infections-ProtocolMichael KaoNo ratings yet
- Bacterial Infections of The Central Nervous System: Paul A. Lapenna, Do Karen L. Roos, MDDocument9 pagesBacterial Infections of The Central Nervous System: Paul A. Lapenna, Do Karen L. Roos, MDOver HidalgoNo ratings yet
- Infecciones Por Arbovirus - ContinuumDocument13 pagesInfecciones Por Arbovirus - ContinuumNeurología RebagliatiNo ratings yet
- AbcesoDocument2 pagesAbcesoRocio LedesmaNo ratings yet
- Brain Abscess: State-Of-The-Art Clinical ArticleDocument17 pagesBrain Abscess: State-Of-The-Art Clinical ArticleAwais KhanNo ratings yet
- Brain Abscess: State-Of-The-Art Clinical ArticleDocument17 pagesBrain Abscess: State-Of-The-Art Clinical ArticleIqbal AbdillahNo ratings yet
- Infecciones Virales Del SNCDocument246 pagesInfecciones Virales Del SNCpediatria hncase100% (1)
- Mold Infections of The Central Nervous SystemDocument15 pagesMold Infections of The Central Nervous SystemJohn TusselNo ratings yet
- Noor2016 PDFDocument13 pagesNoor2016 PDFBilel DhaouadiNo ratings yet
- Roos, Karen L. Greenlee, John E. - Meningitis and EncephalitisDocument14 pagesRoos, Karen L. Greenlee, John E. - Meningitis and EncephalitisNadila Nur PratiwiNo ratings yet
- And Surgical Drainage: Case Report and Literature Review of Central Nervous System PseudallescheriasisDocument5 pagesAnd Surgical Drainage: Case Report and Literature Review of Central Nervous System PseudallescheriasisMarcela PalendengNo ratings yet
- Covid-19 mRNA VaccinesDocument16 pagesCovid-19 mRNA VaccinesSargent Goodchild100% (2)
- Second Thoughts About Viruses Vaccines and The HIV Part 2 Robert.O.YoungDocument5 pagesSecond Thoughts About Viruses Vaccines and The HIV Part 2 Robert.O.YoungDavidNo ratings yet
- Emergency Department Management of Adults With Infectious MeningitisDocument24 pagesEmergency Department Management of Adults With Infectious MeningitisJuan Carlos Navarro GarcíaNo ratings yet
- Sphingomonas KoreensisDocument11 pagesSphingomonas KoreensisSMIBA MedicinaNo ratings yet
- Review: Nanovaccines: Recent Developments in VaccinationDocument9 pagesReview: Nanovaccines: Recent Developments in VaccinationGina CubillasNo ratings yet
- Meningitis Thesis StatementDocument9 pagesMeningitis Thesis StatementMichele Thomas100% (1)
- Dechlorane Plus and Related Compounds in Peregrine Falcon (Falco Peregrinus) Eggs From Canada and SpainDocument6 pagesDechlorane Plus and Related Compounds in Peregrine Falcon (Falco Peregrinus) Eggs From Canada and SpainlacosNo ratings yet
- Nihms 1713249Document13 pagesNihms 1713249Achilles Fkundana18No ratings yet
- JNeurosciRuralPract 2013 4 5 67 116472Document17 pagesJNeurosciRuralPract 2013 4 5 67 116472Silvia EmyNo ratings yet
- Sepsis in 2018: A Review: Learning ObjectivesDocument8 pagesSepsis in 2018: A Review: Learning ObjectivesViky DamayNo ratings yet
- JNeurosciRuralPract 2013 4 5 67 116472Document17 pagesJNeurosciRuralPract 2013 4 5 67 116472lathifatulNo ratings yet
- Hussain 2024Document6 pagesHussain 2024jose mendozaNo ratings yet
- Bodil Sen 2018Document15 pagesBodil Sen 2018Sannita Mayusda BadiriNo ratings yet
- TuberculomaDocument4 pagesTuberculomalittlecandiesNo ratings yet
- InfectiousmeningitisDocument10 pagesInfectiousmeningitisHow ToNo ratings yet
- Guidelines For Healthcare-Associated VentriculitisDocument32 pagesGuidelines For Healthcare-Associated VentriculitisDaniel Martínez CruzNo ratings yet
- Immunology Science and Public Health TheDocument1 pageImmunology Science and Public Health ThezishidayatullahbatamNo ratings yet
- Jurnal PendukungDocument9 pagesJurnal PendukungBrillyant S RaintamaNo ratings yet
- 2017 Infectious Diseases Society of America's Clinical Practice Guidelines For Healthcare-Associated Ventriculitis and MeningitisDocument32 pages2017 Infectious Diseases Society of America's Clinical Practice Guidelines For Healthcare-Associated Ventriculitis and MeningitisMatus AlbertoNo ratings yet
- Bacterial Brain Abscess: Kevin Patel, MD, and David B. Clifford, MDDocument9 pagesBacterial Brain Abscess: Kevin Patel, MD, and David B. Clifford, MDRiskalFebriaryNo ratings yet
- REFERAT 1-S2.0-S1201971210023684-MainDocument14 pagesREFERAT 1-S2.0-S1201971210023684-MainVantyNo ratings yet
- Bacterial Brain Abscess: Kevin Patel, MD, and David B. Clifford, MDDocument9 pagesBacterial Brain Abscess: Kevin Patel, MD, and David B. Clifford, MDDayuKurnia DewantiNo ratings yet
- Original Papers: Bacterial Brain Abscess: Microbiological Features, Epidemiological Trends and Therapeutic OutcomesDocument9 pagesOriginal Papers: Bacterial Brain Abscess: Microbiological Features, Epidemiological Trends and Therapeutic OutcomesIqbal AbdillahNo ratings yet
- ALS-Rot-4-meningococcemia (MOJICA, NOAH KENT)Document18 pagesALS-Rot-4-meningococcemia (MOJICA, NOAH KENT)Noah Kent MojicaNo ratings yet
- Community-Acquired Bacterial Meningitis (Van de Beek)Document13 pagesCommunity-Acquired Bacterial Meningitis (Van de Beek)gaz.med20No ratings yet
- Fnmol 12 00057Document9 pagesFnmol 12 00057Uziko 10No ratings yet
- Bacterial MeningitisDocument26 pagesBacterial MeningitisRai HannaNo ratings yet
- NeurocisticercosisDocument4 pagesNeurocisticercosisCarlos MendozaNo ratings yet
- Device-Associated Central Nervous System Infection Caused by Candida ParapsilosisDocument6 pagesDevice-Associated Central Nervous System Infection Caused by Candida ParapsilosisMaria ObrejaNo ratings yet
- Nobel Medicine 2023Document3 pagesNobel Medicine 2023suma ethukuNo ratings yet
- Ciw 861Document32 pagesCiw 861Dr Vikas GuptaNo ratings yet
- Shapiro L 2021Document4 pagesShapiro L 2021Vlad VochituNo ratings yet
- Atb Meningitis Coid 2018Document12 pagesAtb Meningitis Coid 2018Danny JacobusNo ratings yet
- Central Nervous System Tuberculosis: John M. LeonardDocument10 pagesCentral Nervous System Tuberculosis: John M. LeonardMarcela Catalina Fandiño VargasNo ratings yet
- Huttunenetal ActaPaediatr2009Document8 pagesHuttunenetal ActaPaediatr2009Nathasya B.W.No ratings yet
- MeningitisDocument17 pagesMeningitisSanti IsmatulNo ratings yet
- IDSA Meningitis GuidelinesDocument18 pagesIDSA Meningitis GuidelinesSahid López GarcíaNo ratings yet
- Risk Assessment in Infection Control Which RisksDocument2 pagesRisk Assessment in Infection Control Which RiskspedroaorNo ratings yet
- Bacterial Brain Abscess: An Outline For Diagnosis and ManagementDocument10 pagesBacterial Brain Abscess: An Outline For Diagnosis and ManagementWIWI HRNo ratings yet
- MeningitisDocument8 pagesMeningitismeksyNo ratings yet
- Post-Viral Effects of COVID-19 in The Olfactory System and Their ImplicationsDocument9 pagesPost-Viral Effects of COVID-19 in The Olfactory System and Their Implicationschan kyoNo ratings yet
- Pediatric Neurology: Topical ReviewDocument17 pagesPediatric Neurology: Topical ReviewclaudyNo ratings yet
- DexametasonDocument7 pagesDexametasonKeniIONo ratings yet
- Next-Generation Vaccines: Nanoparticle-Mediated DNA and mRNA DeliveryDocument17 pagesNext-Generation Vaccines: Nanoparticle-Mediated DNA and mRNA DeliveryKaren BurgosNo ratings yet
- 1 s2.0 S1473309916303851 MainDocument11 pages1 s2.0 S1473309916303851 Mainliz zuñigaNo ratings yet
- Epidemiology and Disease Burden of Hospitalized Children With Viral Central Nervous System Infections in China, 2016 To 2020Document7 pagesEpidemiology and Disease Burden of Hospitalized Children With Viral Central Nervous System Infections in China, 2016 To 2020chenxp1111No ratings yet
- Bacterial Brain AbscessDocument10 pagesBacterial Brain AbscessTheroux delucNo ratings yet
- Public Beliefs On Antibiotics and Respiratory Tract Infections: An Internet-Based Questionnaire StudyDocument7 pagesPublic Beliefs On Antibiotics and Respiratory Tract Infections: An Internet-Based Questionnaire StudyxyzNo ratings yet
- EHRA-new Oral AnticoagulantDocument13 pagesEHRA-new Oral AnticoagulantBush HsiehNo ratings yet
- Cefwef Epo Fefaef EfDocument15 pagesCefwef Epo Fefaef EfBush HsiehNo ratings yet
- Intracerebral HemorrhageDocument39 pagesIntracerebral HemorrhageBush HsiehNo ratings yet
- UKPDS Brain Bank PD CriteriaDocument1 pageUKPDS Brain Bank PD CriteriaBush HsiehNo ratings yet
- Lets Learn About Germs-2Document4 pagesLets Learn About Germs-2api-506606579No ratings yet
- The National Academies Press: What You Need To Know About Infectious Disease (2011)Document45 pagesThe National Academies Press: What You Need To Know About Infectious Disease (2011)Robert EnteenNo ratings yet
- Combine PDFDocument91 pagesCombine PDFIbrahim SawaftaNo ratings yet
- 09 Apr 2021: UPSC Exam Comprehensive News Analysis: A. GS 1 Related B. GS 2 RelatedDocument12 pages09 Apr 2021: UPSC Exam Comprehensive News Analysis: A. GS 1 Related B. GS 2 RelatedArpita Sen BhattacharyaNo ratings yet
- Biology Investigatory ProjectDocument14 pagesBiology Investigatory Projectdesaipiyush1234No ratings yet
- Epidemiology Modeling With StellaDocument33 pagesEpidemiology Modeling With Stellaqiuyan.yuanNo ratings yet
- Severe Acute Respiratory Syndrome: Dadam Revathi ReddyDocument23 pagesSevere Acute Respiratory Syndrome: Dadam Revathi Reddyrevathidadam55555No ratings yet
- Microbiology Research ProposalDocument20 pagesMicrobiology Research ProposalSalman KhanNo ratings yet
- HLT37215 Cert III Pathology Collection Brochure v1 2022Document7 pagesHLT37215 Cert III Pathology Collection Brochure v1 2022aman_grewal03mNo ratings yet
- ImmunityDocument29 pagesImmunitySHREE SWAMINARAYAN NURSING COLLEGE CHIKHLINo ratings yet
- Lymphatic System Anatomy and Physiology - NurseslabsDocument35 pagesLymphatic System Anatomy and Physiology - NurseslabsAlyssum MarieNo ratings yet
- BS 2229 aHBe 2Document36 pagesBS 2229 aHBe 2Kishor SinghNo ratings yet
- Safety and Efficacy of The BNT162b2 MRNA Covid-19 Vaccine Through 6 MonthsDocument13 pagesSafety and Efficacy of The BNT162b2 MRNA Covid-19 Vaccine Through 6 MonthsChristian GaraffaNo ratings yet
- A Brief Timeline of The Wuhan Lab Dr. Fauci FundedDocument13 pagesA Brief Timeline of The Wuhan Lab Dr. Fauci FundedNguyễn Nhung100% (1)
- VaccinationDocument28 pagesVaccinationM AQIB ASLAMNo ratings yet
- Immulite: Anti-HDocument28 pagesImmulite: Anti-HpinoponiNo ratings yet
- Hazards of Surgical Diathermy PDFDocument6 pagesHazards of Surgical Diathermy PDFKhalidHussain100% (1)
- Chapter 15: Streptococci, Enterococcus, and Other Catalase-Negative, Gram-Positive CocciDocument9 pagesChapter 15: Streptococci, Enterococcus, and Other Catalase-Negative, Gram-Positive CocciJezzah Mae CañeteNo ratings yet
- 5 6300732772877075609Document131 pages5 6300732772877075609Manoranjan sahuNo ratings yet
- Rice Genome ProjectDocument20 pagesRice Genome ProjectRanjith MuralidharanNo ratings yet
- Rapid Increase of A Sar Cov 2 Covid 19 Variant With Multiple Spike Protein MutationsDocument1 pageRapid Increase of A Sar Cov 2 Covid 19 Variant With Multiple Spike Protein MutationsALEJANDRO COUTIÑO VALDOVINOSNo ratings yet
- Prescurtari WhonetDocument6 pagesPrescurtari WhonetSimona TalpesNo ratings yet
- Module 2 - PLANNING (Davao)Document20 pagesModule 2 - PLANNING (Davao)Anne Abejuela ObialNo ratings yet
- Bacteria, Viruses, and Fungi, Oh My!Document37 pagesBacteria, Viruses, and Fungi, Oh My!AkshitaNo ratings yet
- Report HPV, Shingles and Chicken PoxDocument4 pagesReport HPV, Shingles and Chicken PoxJarold PerezNo ratings yet
- An Interview Between A Doctor and A Reporter Took Place To Answer The Question of The Mass About The Current Situation and About The Different Types of CovidDocument2 pagesAn Interview Between A Doctor and A Reporter Took Place To Answer The Question of The Mass About The Current Situation and About The Different Types of CovidJuliene Ann B. BolusoNo ratings yet
- Canine Babesiosis Treatment RegiemsDocument5 pagesCanine Babesiosis Treatment RegiemsHitesh Shandilaya100% (1)
- Virology Journal: Diagnosis of Genital Herpes Simplex Virus Infection in The Clinical LaboratoryDocument31 pagesVirology Journal: Diagnosis of Genital Herpes Simplex Virus Infection in The Clinical LaboratoryEpi PanjaitanNo ratings yet
- Virology Influenza Nad ParainfluenzaDocument4 pagesVirology Influenza Nad ParainfluenzaHisham ChomanyNo ratings yet