Professional Documents
Culture Documents
Naming Reaction Final
Uploaded by
Rajendra Thamerci0 ratings0% found this document useful (0 votes)
23 views9 pagesThe document lists 51 chemical reactions along with their substrates, reagents, and products. It covers reactions involving the conversion of hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids, amines, and aromatic compounds. Some of the most common reactions included are hydrogenation, halogenation, oxidation, reduction, substitution, elimination, addition, condensation, and rearrangement reactions.
Original Description:
Original Title
naming reaction final (2)
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document lists 51 chemical reactions along with their substrates, reagents, and products. It covers reactions involving the conversion of hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids, amines, and aromatic compounds. Some of the most common reactions included are hydrogenation, halogenation, oxidation, reduction, substitution, elimination, addition, condensation, and rearrangement reactions.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
23 views9 pagesNaming Reaction Final
Uploaded by
Rajendra ThamerciThe document lists 51 chemical reactions along with their substrates, reagents, and products. It covers reactions involving the conversion of hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids, amines, and aromatic compounds. Some of the most common reactions included are hydrogenation, halogenation, oxidation, reduction, substitution, elimination, addition, condensation, and rearrangement reactions.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 9
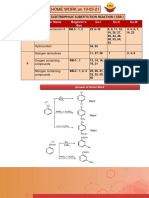
Name Substrate Reagent Product Reaction
1. Sabatier - Senderens Unsaturated Metal catalyst Alkane
Reduction Hydrocarbon ( Raney Ni, Pd, Pt )
2. Wurtz Reeaction Alkyl Halide Na / dry ether Alkane
( R–X ) ( R–R )
3. Corey House Alkyl Halide Gilman’s reagnt Alkane
synthesis ( R–X ) ( R2CuLi ) ( R–R )
4. Wolff Krishner Aldehyde / Ketone i) N2H4 ii) RO–Na+ Alkane
Reduction
5. Clemmensen Aldehyde / Ketone ZnHg / Conc. HCl Alkane
Reduction
6. Decarboxylation Carboxylic acid NaOH + CaO Alkane
Reaction (soda lime)
Name Substrate Reagen Product Reaction
7. Birch Reduction Alkyne Li or Na in liq. NH3 Alkene
8. Ozonolysis Alkene i) O3, ii) H2O/Zn Carbonyl
Compound
9. Wecker Synthesis Alkene Aq. PdCl2 / CuCl2 Aldehyde
10. Kolbe Electrolysis Sodium salt of Electrolysis Alkane /
carboxylic acid Alkene /
Alkyne
11. Finkelstein Reaction Alkyl chloride / NaI / acetone Alkyl Iodide
Alkyl bromide (R– I)
12. Swart Reaction Alkyl chloride / AgF or Hg2F2 or Alkyl fluoride
Alkyl bromide CoF2 or SbF3 (R–F)
Name Substrate Reagent Product Reaction
13. Darzan’s Method Alcohol SOCl2 / Pyridine Alkyl chloride
14. Hunsdicker Silver salt of Cl2 or Br2 in CCl4 Alkyl chloride /
Reaction carboxylic acid Alkyl bromide
(RCOOAg)
15. Haloform Reaction Alcohol and Carbonyl X2 / OH– Haloform (CHX3)
comp.
16. Reimer Teimann Phenol CHCl3/KOH Salicyldehyde
Reaction
17. Carbyl amine 1o Amine CHCl3/KOH Isocyanide
Reaction (Aliphatic or Aromatic) (Bad odour)
18. Hydroboration Alkene/ Alkyne i) B2H6 ii) H2O2/OH– Alcohol
Oxidation (HBO)
Name Substrate Reagent Product Reaction
19. Oxymercuration Alkene / Alkyne i) (CH3COO)2Hg /THF Alcohol /
De-mercuration ii) Redn by NaBH4 Carbonyl
(OMD) compound
20. Bouveault Blanc Aldehyde / Ketone / Na + C2H5OH Alcohol
Reduction Ester
21. MPV Aldehyde / Ketone Aluminium iso propoxide Alcohol
Reduction (AIP)
22. Williamsons Alkyl Halide RO–Na+ (sodium alkoxide) Ether
Ether Synthesis (For best yield 1o)
23. Oxoprocess Alkene CO + H2 / Co2(CO)8 Aldehyde
24. Rosenmund Acid chloride Pd + S + CaCO3 Aldehyde
reaction
Name Substrate Reagent Product Reaction
25. Stephen’s Alkyl Cyanide i) SnCl2 + HCl; ii) H2O Aldehyde
reduction
26. Aldol Aldehyde / Ketone dil. KOH / Aldol / Ketol
Condensation (having –hydrogen) (–hydroxyl
carbonyl
compound)
27. Cannizaro Only aldehyde conc. KOH Potassium salt of
Reaction (no –hydrogen) carboxylic acid
and Alcohol
28. Tischenko Only aldehyde Aluminium ethoxide Ester
reaction (C2H5O)3Al
29. HVZ Reaction Carboxylic acid Cl2 or Br2 / Red P –Halo carboxylic
(having –hydrogen) acid
30. Schmidt Carboxylic acid N3H / H2SO4 1o–Amine
Reaction
31. Hofmann Acid amide Br2 / NaOH 1o–Amine
Hypobromite
reaction
Name Substrate Reagent Product Reaction
32. Gabriel Phthalimide Alkyl halide i) Pot. pthahlimide 1o–Amine
Reaction ii) H2O / H+
33. Hofmann Alkyl Halide NH3 1o / 2o / 3o – Amine
Amonolysis + Qutarnary Ammonium
Process salt
34. Claisen Ester RO–Na+ –keto ester
Condensation (having –hydrogen)
35. Hofmann 1o–Amine CS2 / HgCl2 Alkyl iso thiocyanide
Mustard Oil (RNCS)
reaction
36. Friedel Craft Benzene R–Cl or RCOCl / Alkyl benzene /
Reaction anhyd. AlCl3 Acyl benzene
37. Gattermann Koch Benzene CO + HCl / Benzaldehyde
Reaction Anhyd. AlCl3
Name Substrate Reagent Product Reaction
38. Gattermann Benzene HCN + HCl / Benzaldehyde
Aldehyde Anhyd. AlCl3
Synthesis
39. Wurtz Fittig Halo benzene + RCl Na / dry ehter Alkyl benzene
40. Fittig Halo benzene Na / dry ether Biphenyl
Reaction
41. Ulmann Iodobenzene Cu / Biphenyl
Reaction
42. Etard Alkyl benzene CrO2Cl2 Benzaldehyde
Oxidation
43. Sandmeyer Benzene diazononium CuX + HX Chloro benzene /
Reaction chloride (BDC) (X = Cl, Br, CN) Bromo benzene
Name Substrate Reagent Product Reaction
Cyano benzene
44. Balz Schiemann Benzene HBF4 / Fluoro benzene
Reaction diazononium
chloride (BDC)
45. Dow’s Method Chlorobenzene Aq. NaOH / 300oC/ Phenol
200 atm.
46. Schotten Baumann Phenol / Aniline Benzoyl chloride Ester / Amide
Reaction C6H5COCl
47. Kolbe Schmitt Phenol i) NaOH Salicylic acid
Reaction ii) CO2 / 6 atm /
140oC
48. Laderer Menasse Phenol HCHO / H+ Novolac / Bakelite
Reaction
49. Diazotization Aniline NaNO2 + HCl Benzene diazonium
reaction (0–5oC) chloride(BDC)
Name Substrate Reagent Product Reaction
50. Benzoin Benzaldehyde Alc. KCN Benzoin
Condensation
51. Perkin Reaction Benzaldehyde Acetic anhydride / Cinemic acid
Sodium acetate (,–unsaturated acid)
You might also like
- Annual Reports in Organic Synthesis — 1971From EverandAnnual Reports in Organic Synthesis — 1971John McMurryNo ratings yet
- Named Reaction Cheatsheet For Organic Chemistry by MeritnationDocument2 pagesNamed Reaction Cheatsheet For Organic Chemistry by Meritnationhanushvenkat6No ratings yet
- Annual Reports in Organic Synthesis — 1972From EverandAnnual Reports in Organic Synthesis — 1972John McMurryNo ratings yet
- Solutions For Conversions in Organic ChemistryDocument1 pageSolutions For Conversions in Organic ChemistryNIMISH MUTYAPUNo ratings yet
- NamereactionorganicDocument13 pagesNamereactionorganicdeykrishna654100% (1)
- Named RXNDocument10 pagesNamed RXNssatechies62No ratings yet
- Namedreactions H: Aloalkanesandhaloarenes 1Document11 pagesNamedreactions H: Aloalkanesandhaloarenes 1Vishant SinghNo ratings yet
- NotesDocument10 pagesNotespovikanimathiNo ratings yet
- Important Reagents TreDocument3 pagesImportant Reagents Treraghava123456No ratings yet
- Aldehydes & Ketones MKA SIRDocument51 pagesAldehydes & Ketones MKA SIRcrawlskullNo ratings yet
- Important Organic Chemistry ReactionsDocument5 pagesImportant Organic Chemistry ReactionsK VIKASNo ratings yet
- 51 Reactions From PDFDocument5 pages51 Reactions From PDFAbhinandan Sinha33% (3)
- Named ReactionsDocument5 pagesNamed ReactionsJoshuaNo ratings yet
- 12th - Genral - Named Organic Reaction Sheet Class NotesDocument5 pages12th - Genral - Named Organic Reaction Sheet Class Notesaaravtrivedi313No ratings yet
- Name Reactions of Organic ChemistryDocument7 pagesName Reactions of Organic Chemistryaashishdevkota185No ratings yet
- Edexcel IAL Chemistry A-Level: Topic 20: Organic SynthesisDocument8 pagesEdexcel IAL Chemistry A-Level: Topic 20: Organic SynthesisCornflake 25No ratings yet
- Crux and Reagents of Organic ChemDocument4 pagesCrux and Reagents of Organic ChemBILL RUSSO100% (4)
- NCERT Important Name Reactions For RevisionDocument34 pagesNCERT Important Name Reactions For Revisionyimisa2927No ratings yet
- Organic SynthesisDocument1 pageOrganic Synthesiszozoxo0% (1)
- Name Reactions of Organic ChemistryDocument7 pagesName Reactions of Organic ChemistryNaynam SharmaNo ratings yet
- Chemical Test Orgnic Chemistry 2020Document4 pagesChemical Test Orgnic Chemistry 2020Mukesh GanjawalaNo ratings yet
- Pdf-Haloalkanes and HaloarenesDocument159 pagesPdf-Haloalkanes and HaloarenesOmkar Singh Shekhawat100% (2)
- Class 12 Organic Name ReacyionDocument7 pagesClass 12 Organic Name Reacyioncyeditz10101No ratings yet
- 45 Hydrocarbons AlkanesDocument7 pages45 Hydrocarbons Alkanessujalgupta0123456789No ratings yet
- Chemical Test To Distinguish Between Pair of Organic CompoundDocument11 pagesChemical Test To Distinguish Between Pair of Organic CompoundHishq Dhiman100% (1)
- All Named Reactions of ChemistryDocument11 pagesAll Named Reactions of ChemistryAbhay Narayan Mishra0% (1)
- Organic Flow Chart MrSyed PDF NewDocument1 pageOrganic Flow Chart MrSyed PDF Newmuhammadim2007No ratings yet
- Chem 12 Organic DistinguishDocument5 pagesChem 12 Organic DistinguishNabaratna Biswal0% (1)
- Organic Named ReactionsDocument21 pagesOrganic Named ReactionsP. Phani prasadNo ratings yet
- Chapter 19. Aldehydes and Ketones: Nucleophilic Addition ReactionsDocument98 pagesChapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions張湧浩No ratings yet
- Name Reaction Organic PDFDocument41 pagesName Reaction Organic PDFPummy Thakur100% (1)
- Summary of OChem ReactionsDocument21 pagesSummary of OChem ReactionsSelina YangNo ratings yet
- Organic Chemistry Fiitjee Flowcharts PDFDocument12 pagesOrganic Chemistry Fiitjee Flowcharts PDFAkshit Sharma50% (4)
- Chemical Reactions of Alkanes: Mechanism Reaction Reagent Condition Catalysts Product(s)Document9 pagesChemical Reactions of Alkanes: Mechanism Reaction Reagent Condition Catalysts Product(s)Sam LeeNo ratings yet
- 738616703organic ConversionDocument8 pages738616703organic ConversionSakshi SinghNo ratings yet
- CHEM F311 Lecture 2 Oxiding and Reducing AgentsDocument16 pagesCHEM F311 Lecture 2 Oxiding and Reducing AgentsSAYAN RAYNo ratings yet
- Aldehydes and KetonesDocument25 pagesAldehydes and KetonesPatricia DinaNo ratings yet
- Summary of Organic Reactions: Reaction FormatDocument3 pagesSummary of Organic Reactions: Reaction FormatKim CNo ratings yet
- Hasan Sayginel: Edexcel A Level Organic ChemistryDocument41 pagesHasan Sayginel: Edexcel A Level Organic ChemistryDEEBANNo ratings yet
- Hydrocarbons SheetDocument38 pagesHydrocarbons Sheetpathalam subrahmanyamNo ratings yet
- Full NCERT Organic Reactions ch10 to ch14 - इसे करके जाओगे तो 65+ Score पक्का (25.2.2021)Document89 pagesFull NCERT Organic Reactions ch10 to ch14 - इसे करके जाओगे तो 65+ Score पक्का (25.2.2021)Sameer Narula100% (1)
- Comman Reagents Org. ChemistryDocument3 pagesComman Reagents Org. ChemistryPoornima RaviNo ratings yet
- Aldehydes Ketones Carboxylic AcidsDocument119 pagesAldehydes Ketones Carboxylic AcidsKashvi KhandelwalNo ratings yet
- Organic Chemistry: Name ReactionsDocument7 pagesOrganic Chemistry: Name ReactionspiyashnathNo ratings yet
- Slide 1Document26 pagesSlide 1ShreyaNo ratings yet
- CAIE Chemistry A-Level: 21: Organic SynthesisDocument4 pagesCAIE Chemistry A-Level: 21: Organic SynthesisahumanbeinginearthNo ratings yet
- Name ReactionDocument15 pagesName Reactionnirbhay shukla100% (1)
- Organic SynthesisDocument6 pagesOrganic Synthesiszarfan sabriNo ratings yet
- Complete Organic Chemistry (Brahmastra) Part 2Document763 pagesComplete Organic Chemistry (Brahmastra) Part 2mohdamaankhan74No ratings yet
- Hydrocarbons Formula SheetDocument27 pagesHydrocarbons Formula SheetADARSH SINGHNo ratings yet
- Post Lab Expt 11 15Document33 pagesPost Lab Expt 11 15Ryle AquinoNo ratings yet
- FN 2Document3 pagesFN 2idon'tgiveachogiwaNo ratings yet
- Organic Chemistry ReactionDocument3 pagesOrganic Chemistry ReactionGAMEPORIUMNo ratings yet
- Organic Chem Reactions: 1. AlkanesDocument6 pagesOrganic Chem Reactions: 1. AlkanesFatema KhatunNo ratings yet
- FSPJFSPJSFPJFSPJFSPJDocument6 pagesFSPJFSPJSFPJFSPJFSPJFatema KhatunNo ratings yet
- Reaction Summary: ALKENES: Reagent Conditions Products Observations Example/DiagramDocument1 pageReaction Summary: ALKENES: Reagent Conditions Products Observations Example/DiagramVictoria KairooNo ratings yet
- All Name Reactions of Chemistry Class 12th Cbse & Isc PDFDocument11 pagesAll Name Reactions of Chemistry Class 12th Cbse & Isc PDFprabs2006917881% (145)
- Reduction, Oxidation - Hydrolysis Theory PDFDocument14 pagesReduction, Oxidation - Hydrolysis Theory PDFGOURISH AGRAWALNo ratings yet
- All Name Reactions of Chemistry Class 12th Cbse & Isc PDFDocument11 pagesAll Name Reactions of Chemistry Class 12th Cbse & Isc PDFzakiya100% (2)
- 2018-May ECD-221 25Document1 page2018-May ECD-221 25Rajendra ThamerciNo ratings yet
- 2020-Dec ECD-314 1Document1 page2020-Dec ECD-314 1Rajendra ThamerciNo ratings yet
- The Nyquist Plot A Frequency Response Analysis TechniqueDocument33 pagesThe Nyquist Plot A Frequency Response Analysis TechniqueRajendra ThamerciNo ratings yet
- 2019-Dec ECD-314 30Document2 pages2019-Dec ECD-314 30Rajendra ThamerciNo ratings yet
- 2020-Dec ECD-314 1Document1 page2020-Dec ECD-314 1Rajendra ThamerciNo ratings yet
- 2018-Dec EC-3504 249Document2 pages2018-Dec EC-3504 249Rajendra ThamerciNo ratings yet
- DITFFTDocument3 pagesDITFFTRajendra ThamerciNo ratings yet
- Chapter 7Document29 pagesChapter 7Rajendra ThamerciNo ratings yet
- Important NoteDocument1 pageImportant NoteRajendra ThamerciNo ratings yet
- N Elemant ArrayDocument14 pagesN Elemant ArrayRajendra ThamerciNo ratings yet
- Jagadguru Adi Sankara - enDocument47 pagesJagadguru Adi Sankara - enRajendra ThamerciNo ratings yet
- B.tech (C) - I (Ece - 101 Paper - Mathematics - I)Document3 pagesB.tech (C) - I (Ece - 101 Paper - Mathematics - I)Rajendra ThamerciNo ratings yet
- Magnetic Materials For MEMSDocument4 pagesMagnetic Materials For MEMSRajendra ThamerciNo ratings yet
- Rajendra: Analog (Onmu (Document15 pagesRajendra: Analog (Onmu (Rajendra ThamerciNo ratings yet
- 160.8.8 Virtual Work For Beams Example SupportDocument17 pages160.8.8 Virtual Work For Beams Example SupportArnold Magdalita FranciscoNo ratings yet
- Chapter 7 IntegralsDocument216 pagesChapter 7 IntegralsRishabh SharmaNo ratings yet
- Experiment 7Document3 pagesExperiment 7Ana RodriguesNo ratings yet
- Aldehydes, Ketones, and Carboxylic AcidsDocument39 pagesAldehydes, Ketones, and Carboxylic AcidsashathtNo ratings yet
- Module 2 Types of Organic SubstancesDocument12 pagesModule 2 Types of Organic SubstancesAurora corpuzNo ratings yet
- Chemistry Study Note Revision ExportDocument81 pagesChemistry Study Note Revision ExportsuhxbcnjxksjxnNo ratings yet
- Module 5 Review of Basic Organic CompoundsDocument18 pagesModule 5 Review of Basic Organic CompoundsBig BrotherNo ratings yet
- Alcohols, Phenols & EthersDocument12 pagesAlcohols, Phenols & EtherssiddharthchillapwarNo ratings yet
- Evonik Products CatalogDocument43 pagesEvonik Products Catalog李雷No ratings yet
- Chemistry Alcohols and PhenolsDocument64 pagesChemistry Alcohols and PhenolsM.G.MrithyunjhaiNo ratings yet
- Full Download Book Loose Leaf For Organic Chemistry With Biological Topics PDFDocument41 pagesFull Download Book Loose Leaf For Organic Chemistry With Biological Topics PDFannie.tetreault806100% (14)
- Chemistry - Haloalkanes and HaloarenesDocument34 pagesChemistry - Haloalkanes and HaloarenesKaran VermaNo ratings yet
- Tabela - Pavia 46 - 48Document3 pagesTabela - Pavia 46 - 48João GabrielNo ratings yet
- Midyear Assessment General Chemistry 1Document7 pagesMidyear Assessment General Chemistry 1Jabeguero Marvelyn JessicaNo ratings yet
- PUC II Chemistry Passing PackageDocument28 pagesPUC II Chemistry Passing PackageVaishnavi Vaishu100% (1)
- Module 1Document25 pagesModule 1nicolas.princessmiracleb.kldNo ratings yet
- Chapter IX Alcohols and PhenolsDocument89 pagesChapter IX Alcohols and PhenolsDuy Anh ĐàoNo ratings yet
- Chemistry U3 19-23 CombinedDocument152 pagesChemistry U3 19-23 CombinedHalal BoiNo ratings yet
- Aldehydes and KetonesDocument4 pagesAldehydes and Ketonesnvmohankumar85No ratings yet
- Thiols, Ethers, and SulfidesDocument56 pagesThiols, Ethers, and Sulfidesgsy2023-9150-52879No ratings yet
- 12th Chemistry Syllabus (2023-24)Document7 pages12th Chemistry Syllabus (2023-24)ts397199No ratings yet
- Chemical Weekly May03Document238 pagesChemical Weekly May03devangNo ratings yet
- Organic Compounds, Classification and Properties: For General Chemistry 1/ Grade 12 Quarter 2 / Week 6Document14 pagesOrganic Compounds, Classification and Properties: For General Chemistry 1/ Grade 12 Quarter 2 / Week 6ariinnggg onichaNo ratings yet
- Glandz Product ListDocument24 pagesGlandz Product ListRahul PambharNo ratings yet
- Organic Chemistry Pt.2Document15 pagesOrganic Chemistry Pt.2kiwisareforthesoulNo ratings yet
- IUPAC ExerciseDocument27 pagesIUPAC ExerciseDhritismita KalitaNo ratings yet
- Dwnload Full Biochemistry 4th Edition Mathews Test Bank PDFDocument12 pagesDwnload Full Biochemistry 4th Edition Mathews Test Bank PDFmahoutcawk.cn3ec8100% (14)
- CHEM2311 Syllabus FALL 2022Document12 pagesCHEM2311 Syllabus FALL 2022Rachel MuhlesteinNo ratings yet
- Organic Chemistry Functional Groups and The Molecules of BiochemistryDocument34 pagesOrganic Chemistry Functional Groups and The Molecules of BiochemistryAdriana RodriguezNo ratings yet
- 1 s2.0 S0960894X2300241X MainDocument10 pages1 s2.0 S0960894X2300241X Mainc4ph5s5fjrNo ratings yet
- Ufi-String D PDFDocument4 pagesUfi-String D PDFNermeen ElmelegaeNo ratings yet
- Iit Jee Advanced SyllabusDocument17 pagesIit Jee Advanced SyllabusIITIAN SANJEEV[IITK]No ratings yet
- Organic Chemistry MasterDocument128 pagesOrganic Chemistry MasterLeigh DensingNo ratings yet