Professional Documents
Culture Documents
Distillation & Filtration: Popular Separation Techniques
Uploaded by
Anne FloraOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Distillation & Filtration: Popular Separation Techniques
Uploaded by
Anne FloraCopyright:
Available Formats
Score:
SCIENCE
Distillation -This technique involves boiling and condensation. In this
technique, the mixture is heated until one of the components boils evaporates.
Then the vapor is cooled and changed back into liquid form. The pure liquid
formed after distillation is called the distillate.
Filtration - A separation technique used to separate the components of a
mixture containing an in dissolved solid in a liquid. Filtration may be done cold
or hot, using gravity or applying vacuum, using a Buchner or Hirsch funnel or
a simple glass funnel. The exact method used depends on the purpose of the
filtration, whether it is for the isolation of a solid from a mixture or removal of
impurities from a mixtures.
Evaporation - A technique that is used in separating a mixture, usually a
solution of a solvent and a soluble solid. In this method, the solution is heated
until the organic solvent evaporates where it turns into a gas and mostly leaves
behind the solid residue.
Sieving - A mixture that contains solids of varying size can be separated by
sieving. In this technique, a sieve is used to separate the larger solid from
smaller or finer ones. Separation takes place due to the difference in the size of
particles.
Decantation - Mixtures that contain a solid and a liquid, in which the solid
does not dissolve, can be separated by decantation. In this technique, the
insoluble solid is allowed to settle at the bottom of the mixture. Then the liquid
is poured out, leaving behind the solid.
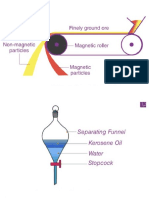
Magnetic Attraction - Mixtures that have components with magnetic
properties can be separated by magnetic attraction. Iron, nickel, and cobalt are
magnetic metals. When any of these metals are combined with non-magnetic
materials, they can be separated by using a magnet.
Chromatography - is an analytical technique commonly used for separating a
mixture of chemical substances into its individual components, so that the
individual components can be thoroughly analyzed.
Winnowing - When the grains are collected from the process of threshing, it
needs to be cleared out of husks and chaffs before it is turned into flour.
Normally the separation of the mixture is carried out with the help of wind or
blowing air. The husk and chaff are blown away by the strong wind when the
farmers drop the mixture from a certain height to the ground. The heavier
grains are collected at one place.
Centrifugation - is a method of separating molecules having different densities
by spinning them in solution around an axis (in a centrifuge rotor) at high
speed? It is one of the most useful and frequently employed techniques in the
molecular biology laboratory.
Sedimentation - is the process of allowing particles in suspension in water
settle out of the suspension under the effect gravity.
Separatory funnel - separation funnel, separating funnel, colloquially sep
funnel, is a piece of laboratory glassware used in liquid-liquid extractions to
separate the components of a mixture into two immiscible solvent phases of
different densities.
Paper Chromatography - technique for separating dissolved chemical
substances by taking advantage of their different rates of migration across
sheets of paper. It is an inexpensive but powerful analytical tool that requires
very small quantities of material.
Threshing - is the process of separating grain from the chaff. In this process, a
mixture of wheat and husk is dropped from a height. After that, the husk is
carried by the wind and forms a heap at some distance away. The husk being
lighter is carried away by the wind and forms a different heap.
Column Chromatography - is used to isolate active ingredients. It is very
helpful in separating compound mixtures. It is used to determine drug
estimation from drug formulations. It is used to remove impurities.
You might also like
- Concept Notes 2 Simple Separation Techniques: EvaporationDocument29 pagesConcept Notes 2 Simple Separation Techniques: EvaporationAya CayananNo ratings yet
- Methods of SeparationDocument8 pagesMethods of SeparationJayson DayaoNo ratings yet
- H?V Dgghwvfj8Xs: Classification of MatterDocument60 pagesH?V Dgghwvfj8Xs: Classification of MatterCheri BulahanNo ratings yet
- Ways of Separating MixturesDocument5 pagesWays of Separating MixturesMay Anne AlmarioNo ratings yet
- Separation Techniques: MagnetismDocument3 pagesSeparation Techniques: MagnetismJohnlloyd NageraNo ratings yet
- Pengantar Metode PemisahanDocument43 pagesPengantar Metode PemisahanMili APRILIANo ratings yet
- Different methods for separating mixturesDocument2 pagesDifferent methods for separating mixturesClark Hailie Wayne EstrellaNo ratings yet
- Separating MixturesDocument5 pagesSeparating Mixturesnyentuk jjangNo ratings yet
- Genchem 1Document2 pagesGenchem 1Káte BenegildoNo ratings yet
- Centrifugation Techniques ExplainedDocument31 pagesCentrifugation Techniques ExplainedZaid YahyaNo ratings yet
- Separation Technique - Pharm Eng IDocument43 pagesSeparation Technique - Pharm Eng Ihakita86No ratings yet
- Chemistry 2 - Separating MixturesDocument7 pagesChemistry 2 - Separating MixturesNaseeb AliNo ratings yet
- " Seperation Process": Seminar OnDocument28 pages" Seperation Process": Seminar OnKabilanNo ratings yet
- Separation Techniques Part 2Document45 pagesSeparation Techniques Part 2Maan Joy Revelo GallosNo ratings yet
- Chromatography Is Used To Separate Mixtures of Substances Into Their ComponentsDocument8 pagesChromatography Is Used To Separate Mixtures of Substances Into Their ComponentsAyrea Riclye Sanaes'yumealoverNo ratings yet
- 8TH GRADE SEPARATING MIXTURESDocument12 pages8TH GRADE SEPARATING MIXTURESKolade Fatai OpeyemiNo ratings yet
- Filtration, EvaporationDocument3 pagesFiltration, EvaporationH.Mohammad JohanyNo ratings yet
- Separating MixturesDocument13 pagesSeparating Mixturesver_at_workNo ratings yet
- Lesson 2 Nothing Last ForeverDocument19 pagesLesson 2 Nothing Last ForeverFlorenda Sorilla SampianoNo ratings yet
- Mechanical Separation AssignmentDocument9 pagesMechanical Separation AssignmentMuhammad UsamaNo ratings yet
- Separating Mixtures: Key Separation MethodsDocument5 pagesSeparating Mixtures: Key Separation MethodsJoelle SwaisNo ratings yet
- Separation TechniquesDocument7 pagesSeparation TechniquesPriyanka WadhwaniNo ratings yet
- Separation Techniques for MixturesDocument22 pagesSeparation Techniques for MixturesJemaicah AbaoNo ratings yet
- SCH 410 Lecture One 2023Document20 pagesSCH 410 Lecture One 2023Samuel MutisyaNo ratings yet
- 2 Notes SEARATING MIXTURESDocument3 pages2 Notes SEARATING MIXTUREScatherine renanteNo ratings yet
- Science: Seperating MixturesDocument6 pagesScience: Seperating MixturesKeith GatbontonNo ratings yet
- Ruth Separatingmixtures 170625071108Document23 pagesRuth Separatingmixtures 170625071108Angeline Libunao NecorNo ratings yet
- Separation of SubstancesDocument5 pagesSeparation of SubstancesdrakbarbabNo ratings yet
- Methods and Equipment for Separating SolidsDocument19 pagesMethods and Equipment for Separating SolidsCherry ObiasNo ratings yet
- Chemical separation methodsDocument18 pagesChemical separation methodsMark Heinrich B. ParasNo ratings yet
- L 1.4 - Mixture SeparationDocument17 pagesL 1.4 - Mixture SeparationJhon Emmanuel E. LusicoNo ratings yet
- Separation TechniquesDocument17 pagesSeparation TechniquesSamuel AjanaNo ratings yet
- Centrifugation, Desiccation and LevigationDocument16 pagesCentrifugation, Desiccation and Levigationengr587No ratings yet
- ChemistryDocument41 pagesChemistryAnne kimNo ratings yet
- ChemDocument4 pagesChemBettina Rose GallardoNo ratings yet
- Separating Mixtures (Notes)Document8 pagesSeparating Mixtures (Notes)Nilar SanNo ratings yet
- Separation Methods: Ways To Separate Mixtures - Chapter 3: Matter & Its PropertiesDocument17 pagesSeparation Methods: Ways To Separate Mixtures - Chapter 3: Matter & Its PropertiesKateBarrionEspinosaNo ratings yet
- Separation Methods: Ways To Separate Mixtures - Chapter 3: Matter & Its PropertiesDocument17 pagesSeparation Methods: Ways To Separate Mixtures - Chapter 3: Matter & Its PropertiesNithy's AcademyNo ratings yet
- CH 1.3 Purifying MaterialsDocument12 pagesCH 1.3 Purifying Materialsaayesha.fatima.rajaNo ratings yet
- Ways of Separating MixturesDocument6 pagesWays of Separating MixturesTheresa IgotNo ratings yet
- Methods-of-purifying-Organic-Compounds-2024Document11 pagesMethods-of-purifying-Organic-Compounds-2024speechless720No ratings yet
- Chemical Purification Methods ListDocument8 pagesChemical Purification Methods ListMd.Mehdi MasudNo ratings yet
- Science ReviewerDocument4 pagesScience Reviewerkeishalorenzo13No ratings yet
- Filtration vs Sedimentation: Common Liquid-Solid Separation MethodsDocument13 pagesFiltration vs Sedimentation: Common Liquid-Solid Separation MethodsRoy Vincent MorenoNo ratings yet
- Seperation TechniqueDocument6 pagesSeperation TechniquelindaoeghagharaNo ratings yet
- MELC 1 Use Properties of Matter To Identify Substances and To Separate ThemDocument19 pagesMELC 1 Use Properties of Matter To Identify Substances and To Separate ThemMarinette MedranoNo ratings yet
- Separation and Classification of SolidsDocument23 pagesSeparation and Classification of Solidsalthea aquinoNo ratings yet
- Lesson 3Document3 pagesLesson 3Earl Vincent MergildoNo ratings yet
- Chem Project - KzyDocument4 pagesChem Project - Kzykzy1234No ratings yet
- CentrifugationDocument8 pagesCentrifugation1ds20ch022No ratings yet
- Different Ways to Separate MixturesDocument30 pagesDifferent Ways to Separate MixturesGlena Bilda BarramedaNo ratings yet
- LeachingDocument5 pagesLeachingizlatan910No ratings yet
- SEPARATIONMETHODSDocument2 pagesSEPARATIONMETHODSJulian LuNo ratings yet
- General Chemistry Group 2 Report FinalDocument34 pagesGeneral Chemistry Group 2 Report FinalMara ClaireNo ratings yet
- Research ReportDocument49 pagesResearch ReportDia EliaNo ratings yet
- Separation and Classification of SolidsDocument3 pagesSeparation and Classification of SolidsChristopher YsitNo ratings yet
- Separation ProcessDocument7 pagesSeparation ProcessLina alikhNo ratings yet
- ZJC Form 1-2 Chemistry NotesDocument29 pagesZJC Form 1-2 Chemistry NotesSalome Mupambirei100% (2)
- Oil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksFrom EverandOil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksNo ratings yet
- COMPUTERDocument1 pageCOMPUTERAnne FloraNo ratings yet
- Quiz # 1 ArtsDocument2 pagesQuiz # 1 ArtsAnne FloraNo ratings yet
- QUIZ #2 MATH g1Document2 pagesQUIZ #2 MATH g1Anne FloraNo ratings yet
- Letter MMDocument23 pagesLetter MMAnne FloraNo ratings yet
- Daily Lesson Log (Template)Document3 pagesDaily Lesson Log (Template)Brisky Buyco100% (5)
- Daily Lesson Log (Template)Document3 pagesDaily Lesson Log (Template)Brisky Buyco100% (5)
- Estudio ArminioDocument13 pagesEstudio ArminioJavier LópezNo ratings yet
- CIE Master 2022 (New Master Programme) ENDocument171 pagesCIE Master 2022 (New Master Programme) ENZar MaghustNo ratings yet
- Ritual and Religion Course at University of EdinburghDocument10 pagesRitual and Religion Course at University of EdinburghRenata DC MenezesNo ratings yet
- Valvula Contrabalance CBV1 10 S O A 30Document21 pagesValvula Contrabalance CBV1 10 S O A 30Judith Daza SilvaNo ratings yet
- Inventions Crossword PuzzleDocument2 pagesInventions Crossword PuzzleAimri910% (1)
- BIG-IP Access Policy Manager CustomizationDocument118 pagesBIG-IP Access Policy Manager CustomizationDhananjai SinghNo ratings yet
- TM Journal Class 5 Pharma Trademarks 2018Document1,192 pagesTM Journal Class 5 Pharma Trademarks 2018Tahir LabbeNo ratings yet
- Solar/Wind/Diesel Hybrid Energy System With Battery Storage For Rural ElectrificationDocument15 pagesSolar/Wind/Diesel Hybrid Energy System With Battery Storage For Rural ElectrificationWelde AynaleNo ratings yet
- Salon Lesson Plan 233-1Document7 pagesSalon Lesson Plan 233-1api-264569989No ratings yet
- Telecom Business Management Systems Net ProjectDocument68 pagesTelecom Business Management Systems Net ProjectRahul RaiNo ratings yet
- MC61ADocument5 pagesMC61AAlison Foster100% (1)
- Energy Performance of Hot, DryDocument128 pagesEnergy Performance of Hot, DrySrinu ReddyNo ratings yet
- PreciControl CMV IgG Avidity - Ms - 05942322190.V4.EnDocument2 pagesPreciControl CMV IgG Avidity - Ms - 05942322190.V4.EnARIF AHAMMED PNo ratings yet
- Elah'Im CultureDocument60 pagesElah'Im CultureRichard David DellermanNo ratings yet
- Kendriya Vidyalaya Sangathan: Observation & ReportingDocument6 pagesKendriya Vidyalaya Sangathan: Observation & ReportingSravan KumarNo ratings yet
- DBR Gensets DPL120UK Installation, Operation and Maintenance Manual For YN 513510 (MANUAL-I - 2958658 - 1 - A) - 1Document282 pagesDBR Gensets DPL120UK Installation, Operation and Maintenance Manual For YN 513510 (MANUAL-I - 2958658 - 1 - A) - 1RaymondNo ratings yet
- Parenteral Fluid Therapy: Types of Intravenous SolutionDocument18 pagesParenteral Fluid Therapy: Types of Intravenous SolutionKathleen Joy Costales Magtanong100% (1)
- Oxidation of CopperDocument21 pagesOxidation of CopperAmeen ShahidNo ratings yet
- Foundations Paper MIF PDFDocument70 pagesFoundations Paper MIF PDFBárbara NunesNo ratings yet
- Nitrogen CycleDocument15 pagesNitrogen CycleKenji AlbellarNo ratings yet
- Unmas Ied Lexicon 0Document71 pagesUnmas Ied Lexicon 0Victor AryeeNo ratings yet
- Tadano Hydraulic Rough Terrain Crane TR 350xl 3 560485 Operation Manual 1999 en JPDocument22 pagesTadano Hydraulic Rough Terrain Crane TR 350xl 3 560485 Operation Manual 1999 en JPmarcowens210992apd100% (126)
- ATPL theory summary formulas and guidelines (40 charactersDocument60 pagesATPL theory summary formulas and guidelines (40 charactersJonas Norvidas50% (2)
- Basavarajeshwari Group of Institutions: Self Balancing UnicycleDocument15 pagesBasavarajeshwari Group of Institutions: Self Balancing UnicycleJeerigi DeepikaNo ratings yet
- Table of ContentsDocument2 pagesTable of ContentsPewter VulturelynxNo ratings yet
- LOGIK Fridge Freezer With Water Dispenser LSD55W18 ManualDocument20 pagesLOGIK Fridge Freezer With Water Dispenser LSD55W18 Manualfbunt2777No ratings yet
- Sample Final Exam Larkin AnswersDocument18 pagesSample Final Exam Larkin AnswersLovejot SinghNo ratings yet
- Lauren Tarshis - (I Survived 05) - I Survived The San Francisco Earthquake, 1906Document66 pagesLauren Tarshis - (I Survived 05) - I Survived The San Francisco Earthquake, 1906Olga de Ramos100% (1)
- Exploratory Factor AnalysisDocument170 pagesExploratory Factor AnalysisSatyabrata Behera100% (7)
- I. VHF CommunicationsDocument12 pagesI. VHF CommunicationsSamuel OyelowoNo ratings yet