Professional Documents
Culture Documents
MT1 Distillation L1
Uploaded by
Shiavm Patel0 ratings0% found this document useful (0 votes)
6 views26 pagesThis document provides an overview of distillation and vapor-liquid equilibrium as it relates to separating components in a solution. It defines distillation as separating components based on their distribution between gas and liquid phases. It also introduces vapor-liquid equilibrium diagrams and how they show the relationship between temperature, pressure, and composition of mixtures. Key points include defining bubble points and dew points on these diagrams and how distillation utilizes changes in temperature to drive phase changes between liquid and gas.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides an overview of distillation and vapor-liquid equilibrium as it relates to separating components in a solution. It defines distillation as separating components based on their distribution between gas and liquid phases. It also introduces vapor-liquid equilibrium diagrams and how they show the relationship between temperature, pressure, and composition of mixtures. Key points include defining bubble points and dew points on these diagrams and how distillation utilizes changes in temperature to drive phase changes between liquid and gas.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views26 pagesMT1 Distillation L1
Uploaded by
Shiavm PatelThis document provides an overview of distillation and vapor-liquid equilibrium as it relates to separating components in a solution. It defines distillation as separating components based on their distribution between gas and liquid phases. It also introduces vapor-liquid equilibrium diagrams and how they show the relationship between temperature, pressure, and composition of mixtures. Key points include defining bubble points and dew points on these diagrams and how distillation utilizes changes in temperature to drive phase changes between liquid and gas.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 26
Mass Transfer-I
Distillation
Lecture 1&2
Dr. Hemant Kumar
Department of Chemical Engineering
DDU Nadiad
9/19/2020 Mass transfer-I Dr Hemant Kumar 1
Distillation
Distillation is a method of separating the
components of a solution which depends on
the distribution of the substances between a
gas and a liquid phase, applied to cases where
all components are present in both phases
• New phases are created from original solution by vaporization
or condensation
• Therefore no external substance is added in the mixture to
produce the second phase as in case of absorption
9/19/2020 Mass transfer-I Dr Hemant Kumar 2
Difference between distillation and other
operations
• Separation of solution of common salt and water- is it a
distillation operation: No (salt cannot be vaporized, therefore
not present in both of the phases)
• Separation of components of a liquid solution of ammonia
and water- absorption and distillation both operations are
possible.
In absorption, air is to be used for stripping the solution
with the difficulty of separating the air and ammonia vapor
mixture.
However distillation is possible by evaporation of
solution with repeated vaporization and condensation.
9/19/2020 Mass transfer-I Dr Hemant Kumar 3
Advantage of distillation over other methods
• In distillation the new phase created differs from original in its
heat content, but heat is readily added or removed
Although cost of doing this must be considered
• Whereas absorption or desorption operation depends on the
introduction of a foreign substance provided us a new
solution which in turn may have to be further separated.
9/19/2020 Mass transfer-I Dr Hemant Kumar 4
Limitations of distillation over other methods
• In distillation no other choice than heat addition and removal
is possible. However, methods like absorption or desorption
involves addition of variety of solvents.
• Ex : separation of hydrocarbon gas from a gas mixture, water
is incapable and addition of hydrocarbon oil can solve the
purpose.
• However, distillation does not requires further treatment to
separate the additionally added substance.
9/19/2020 Mass transfer-I Dr Hemant Kumar 5
Vapor liquid Equilibria
• Separation of a mixture by distillation is based on equilibrium
distribution of components between liquid and vapor phases.
• Therefore a knowledge of VLE is essential for understanding
phenomenon of distillation
• Consider the aqueous solution of ethanol in closed tank
• Assume that vapor space does not contain air and space is
filled with ethanol and water vapors only
9/19/2020 Mass transfer-I Dr Hemant Kumar 6
Vapor liquid Equilibria
• Now heating the vessel at const. temp.
• What do we expect to happen ??
Vap: Ethanol +
• Given sufficient time at constant pressure,
water; y*
the system reaches equilibrium
Liq: Ethanol +
• In vapor space, liquid (x) and vapor (y*)
water; x compositions attains a unique value.
• Now increasing the temperature at constant
Heating
pressure will provide new set of conditions x
At const and y* keeping same constant initial
Temp. composition.
• This way T-x-y* diagram is created at const P
and constant initial solution mixture
composition.
9/19/2020 Mass transfer-I Dr Hemant Kumar 7
What is meant by set of conditions
• This is explained by Gibb’s phase rule
F = C-P+2
• In the previous example C=2, P=2 so that degree of freedom
are F=2
• Total number of parameters and variables are 4 i.e. T, P, x and
y*
• To define the system at equilibrium two parameters out of 4
need to be fixed.
• In the previous example system will be fixed if either of two
values are fixed. For example at const T and P, only one set of
value of x and y* are possible.
• VLE constitutes the physical basis for the separation of the
components
9/19/2020 Mass transfer-I Dr Hemant Kumar 8
Pressure-temperature-composition phase diagram for binary
mixture
• VLE constitutes the physical basis for the separation of the
components by distillation
• Successful design of a distillation column is based on the
accuracy of the vapor liquid Equilibria data
• VLE of binary mixture is discussed here.
• Binary mixture means that the liquid component dissolves in
all proportions to form a homogeneous solutions which are
not essentially ideal
• The binary mixture is not showing maximum and minimum
boiling pt phenomena therefore binary mixture is referred
here as ordinary.
9/19/2020 Mass transfer-I Dr Hemant Kumar 9
3-D graphical representation of Equilibria of
binary mixture
9/19/2020 Mass transfer-I Dr Hemant Kumar 10
Vapor-Liquid Equilibria of pure component
9/19/2020 Mass transfer-I Dr Hemant Kumar 11
Pressure-temperature-composition phase diagram for binary mixture
• In a binary mixture of A and B or A-B, component A is referred
as more volatile i.e. vapor of pure component A is higher
than pure component B at all temperatures
• For binary mixture an additional variable concentration must
be considered.
• Unit considered is mole fraction
• x will be mole fraction of the more volatile component A in
liquid
• y* will be the corresponding equilibrium mole fraction of A in
liquid
9/19/2020 Mass transfer-I Dr Hemant Kumar 12
Constant Pressure Equilibria of binary mixture
• The liquid and vapor region between x=0 to x=1 are separated
by double surface as shown in fig (a)
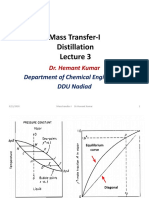
Equilibrium
curve
Diagonal
9/19/2020 Mass transfer-I Dr Hemant Kumar 13
Equilibrium
curve
Diagonal
9/19/2020 Mass transfer-I Dr Hemant Kumar 14
• Binary mixture: Two components A & B
Liquid phase: x (component A) & (1-x) (component B)
Vapor phase: y (component A) & (1-y) (component B)
• x=0 meaning : Liquid composition of more volatile
component A is zero. Therefore this axis represents pure B.
(since axis represents binary mixture)
• x=1 meaning : Liquid composition of more volatile
component A is one. Therefore this axis represents pure A.
• Upper curve represents y* vs t
• Lower curve represents x vs t
• Liquid and vapor mixture at equilibrium are at the same
temperature and pressure throughout, so that the horizontal
tie lines such as line DF join equilibrium mixtures at D and F
• There are an infinite number of tie lines for this diagram
9/19/2020 Mass transfer-I Dr Hemant Kumar 15
Constant Pressure Equilibria of binary mixture
• A mixture on the lower curve as at point D is a saturated liquid
• A mixture on the upper curve at point F is a saturated vapor
• A mixture at E is a two phase mixture consisting of a liquid
phase of composition D and vapor phase of composition at F
in such a way that the average composition of the entire
mixture is represented by E
• Relative amounts of the equilibrium phases are related to the
segments of the tie lines
9/19/2020 Mass transfer-I Dr Hemant Kumar 16
Equilibrium
curve
Diagonal
9/19/2020 Mass transfer-I Dr Hemant Kumar 17
Constant Pressure Equilibria of binary mixture
• Equilibrium in Closed container: which can be kept at constant
pressure by moving a piston
• The solution is entirely liquid
• If it is heated the first bubble of vapor forms at H and has the
composition J, rich in the more volatile substance
• Therefore, the lower curve is called bubble point temperature
curve
• As more of the mixture is vaporized, more of the vapor forms at
the expense of the liquid giving rise to liquid L and its equilibrium
vapor K, although composition of entire mass is still original as at G
• The last drop of liquid vaporizes at M and has the composition at
N
• Superheating the mixture follows path MO
9/19/2020 Mass transfer-I Dr Hemant Kumar 18
Constant Pressure Equilibria of binary mixture
• The mixture has vaporized over a temperature range from H
to M, unlike the single vaporization temperature of pure
substance
• Therefore the term boiling point for a solution ordinarily has
no meaning, since vaporization occurs over a temperature
range
• i.e. from bubble point to dew point
• Open vessel boiling: if a solution like at H is boiled in an open
vessel, with vapors escaping into the atmosphere???
• Since the vapor is richer in more volatile component, the
liquid residue therefore become leaner
• The temperature and composition of the saturated residual
liquid therefore, move along the lower curve toward N as the
distillation proceeds
9/19/2020 Mass transfer-I Dr Hemant Kumar 19
Distribution diagram
• The vapor liquid
equilibrium can also be
shown on a distribution
diagram (x vs y*)
• Point P in the diagram
represents the tie line
DF for example
• Since the vapor is richer
in more volatile
component the curve
lies above 45o diagonal
line
9/19/2020 Mass transfer-I Dr Hemant Kumar 20
Relative volatility:
The greater the distance between the equilibrium curve and
diagonal, the greater the difference in liquid and vapor
composition and easier the separation by distillation
9/19/2020 Mass transfer-I Dr Hemant Kumar 21
Relative volatility
•One relative measure is called separability factor or relative
volatility α
“This is the ratio of the concentration ratio of A and B in one
phase to that in the other phase and is a measure of
separability”
•The value of α changes as x changes from 0 to 1
•If y*= x; except x=0 to x=1, α=1 and no separation is
possible
•The larger the value of α above unity, the greater the
degree of separability
9/19/2020 Mass transfer-I Dr Hemant Kumar 22
VLE at increased pressures
pt5> pt4> pt3> pt2> pt1
9/19/2020 Mass transfer-I Dr Hemant Kumar 23
Constant Temperature Equilibria
9/19/2020 Mass transfer-I Dr Hemant Kumar 24
Constant Temperature Equilibria
•Upper curve is now x vs pt known as bubble point curve
•Lower curve is now dew point curve y* vs pt
•Now liquid solution of binary mixture at point W is
heated at constant temperature in closed vessel and
pressure is reduced
•It will follow path w to R
•pB is the vapor pressure of pure B and pA is the vapor
pressure of pure component A
9/19/2020 Mass transfer-I Dr Hemant Kumar 25
Thanks
9/19/2020 Mass transfer-I Dr Hemant Kumar 26
You might also like
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsFrom EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsRating: 5 out of 5 stars5/5 (1)
- Binary Solid-Liquid DiagramDocument49 pagesBinary Solid-Liquid DiagramMonica NCNo ratings yet
- MT1 Distillation L3Document28 pagesMT1 Distillation L3Shiavm PatelNo ratings yet
- Che 411-Vapour Pressure and BoilingDocument17 pagesChe 411-Vapour Pressure and BoilingHannah CokerNo ratings yet
- CHE333 Simultaneous Heat & Mass Transfer Operations Lecture 4: DistillationDocument59 pagesCHE333 Simultaneous Heat & Mass Transfer Operations Lecture 4: DistillationB MasoomNo ratings yet
- 2018 Distillation NoteDocument120 pages2018 Distillation NoteemmanuelNo ratings yet
- Basics of Distillation: V. K. KapoorDocument62 pagesBasics of Distillation: V. K. Kapoorstardeepakrati100% (1)
- Equilibrium Staged Operations: A1 SlotDocument18 pagesEquilibrium Staged Operations: A1 SlotErmias NigussieNo ratings yet
- Introduction To Multicomponent DistillationDocument13 pagesIntroduction To Multicomponent DistillationKritagyaNo ratings yet
- CMT 405 - Distillation PDFDocument72 pagesCMT 405 - Distillation PDFMuhammad Azri HaziqNo ratings yet
- BCT Module 2Document35 pagesBCT Module 2ValarlaksNo ratings yet
- Distillation L5Document25 pagesDistillation L5Shiavm PatelNo ratings yet
- Chapter 6 7Document47 pagesChapter 6 7Ahbao TiuNo ratings yet
- Lecture 7-DistillationDocument27 pagesLecture 7-DistillationWasim NawazNo ratings yet
- MT1 Distillation L4-6 2021Document29 pagesMT1 Distillation L4-6 2021Smruthi SuvarnaNo ratings yet
- Phase Equilibrium For Separator Design - 0Document52 pagesPhase Equilibrium For Separator Design - 0May May MagluyanNo ratings yet
- Equilibrium Staged Operations: A1 SlotDocument21 pagesEquilibrium Staged Operations: A1 SlotSAI P HARIHARAN 19BCM0030No ratings yet
- Lecture 1 - MSOP - Absorption - 2023Document34 pagesLecture 1 - MSOP - Absorption - 2023Anele HadebeNo ratings yet
- Mass Transfer Fundamentals: Lecture No. 16Document22 pagesMass Transfer Fundamentals: Lecture No. 16Atif MehfoozNo ratings yet
- MT1 Distillation L3 v1Document23 pagesMT1 Distillation L3 v1Shiavm PatelNo ratings yet
- CTDCHA2 - Learning Unit 5 2019Document76 pagesCTDCHA2 - Learning Unit 5 2019Brandon GreenwoodNo ratings yet
- Phase EquilibriaDocument21 pagesPhase EquilibriaGianna Cloe100% (1)
- Unit-1 - FullDocument68 pagesUnit-1 - FullMohammad JunaidNo ratings yet
- Unit II Phase Behaviour of HydrocarbonsDocument68 pagesUnit II Phase Behaviour of HydrocarbonsVishal JadhavNo ratings yet
- Distillation Course Notes 2021Document17 pagesDistillation Course Notes 2021SNo ratings yet
- Lecture 1 - MSOP - Absorption - Added InfoDocument40 pagesLecture 1 - MSOP - Absorption - Added InfoAnele HadebeNo ratings yet
- Distillation IDocument57 pagesDistillation IOsan ThorpeNo ratings yet
- Properties of Pure Substances: Thermodynamics: An Engineering ApproachDocument35 pagesProperties of Pure Substances: Thermodynamics: An Engineering ApproachAsmawi Mohd KhailaniNo ratings yet
- CHME 222 - Lecture 4Document44 pagesCHME 222 - Lecture 4islam.lukmanov2003No ratings yet
- W09 Liquid-Solid Phase DiagramsDocument16 pagesW09 Liquid-Solid Phase DiagramsMar MoncedaNo ratings yet
- CHEG 485 - Lecture 3Document29 pagesCHEG 485 - Lecture 3Muhammad FarooqNo ratings yet
- Mass Transfer - II 3350502: Parth Modi, LecturerDocument39 pagesMass Transfer - II 3350502: Parth Modi, LecturerSMIT CHRISTIANNo ratings yet
- LabManual FinalDocument51 pagesLabManual Finalhos88No ratings yet
- Project Assignment Subject: Physical ChemistryDocument9 pagesProject Assignment Subject: Physical ChemistryBin MậpNo ratings yet
- ME333Ch3 Properties+V1Document89 pagesME333Ch3 Properties+V1marahmansorNo ratings yet
- Chapter 4 - 3&4Document93 pagesChapter 4 - 3&4Nurakmal SyuhAdaNo ratings yet
- Thermodynamics: Chapter 3 - Properties of Pure SubstancesDocument24 pagesThermodynamics: Chapter 3 - Properties of Pure Substancesmmq pakNo ratings yet
- Chapter 1.4 Azeotrope and Multicompnent DistillationDocument29 pagesChapter 1.4 Azeotrope and Multicompnent DistillationAlia TasNo ratings yet
- Chapter Two: Properties of Pure SubstancesDocument62 pagesChapter Two: Properties of Pure SubstancesColorgold BirlieNo ratings yet
- CH 2-BITS F111-S1 2023-24-CMSDocument48 pagesCH 2-BITS F111-S1 2023-24-CMSlipoprotein38No ratings yet
- Distillation PresentationDocument61 pagesDistillation PresentationAli AmjadNo ratings yet
- Introduction To Multi-Component DistillationDocument52 pagesIntroduction To Multi-Component DistillationHussain ShahNo ratings yet
- EQUILUBRIUMDocument31 pagesEQUILUBRIUMcdsingh74No ratings yet
- Two Marks CH 6603 Mass TransferDocument17 pagesTwo Marks CH 6603 Mass Transfersampathkumar100% (1)
- Thermodynamics CHP 2Document56 pagesThermodynamics CHP 2Mysterious WorldNo ratings yet
- G K Agrahari Date: 15/11/2021Document38 pagesG K Agrahari Date: 15/11/2021sidharthNo ratings yet
- Distillation - Lectures 1 To 6 PDFDocument45 pagesDistillation - Lectures 1 To 6 PDFMayank PrasadNo ratings yet
- Lecture 1 - Vapour-Liquid EquilibriumDocument30 pagesLecture 1 - Vapour-Liquid EquilibriumIfiok UsoroNo ratings yet
- DistillatnDocument149 pagesDistillatnVinayak ThalangeNo ratings yet
- MTO2 EXP 3 To 5Document23 pagesMTO2 EXP 3 To 5Par PatelNo ratings yet
- CHE325 Note1 From DR AyoolaDocument30 pagesCHE325 Note1 From DR AyoolaPreciousNo ratings yet
- Separation Process Engineering CHEN 312: Ys18@aub - Edu.lbDocument28 pagesSeparation Process Engineering CHEN 312: Ys18@aub - Edu.lbsoe0303No ratings yet
- Distillation 2Document20 pagesDistillation 2arslanadeelNo ratings yet
- Fluid-Fluid Design ReactorDocument33 pagesFluid-Fluid Design ReactorWendell Kim Llaneta100% (1)
- Mebc Brave 5Document12 pagesMebc Brave 5Laxmi PrasannaNo ratings yet
- Non-Ideal Solutions: Binary SolutionDocument7 pagesNon-Ideal Solutions: Binary SolutionArnab JanaNo ratings yet
- Week 4 Lecture 2Document20 pagesWeek 4 Lecture 2muhammadtalhabilal6666No ratings yet
- Distillation DesignDocument30 pagesDistillation DesignAlonso Flores BelloNo ratings yet
- Chapter 4 - Phase BehaviourDocument80 pagesChapter 4 - Phase BehaviourZulfikri ZulkifliNo ratings yet
- A Shift-12.02.2024 Report1Document1 pageA Shift-12.02.2024 Report1Shiavm PatelNo ratings yet
- MT L19 DistillationDocument32 pagesMT L19 DistillationShiavm PatelNo ratings yet
- Lecture-7 VLE Model-Raoult's LawDocument6 pagesLecture-7 VLE Model-Raoult's LawShiavm PatelNo ratings yet
- Lecture-8,9,10 VLE DiagramsDocument64 pagesLecture-8,9,10 VLE DiagramsShiavm PatelNo ratings yet
- Lecture-5 Gibbs Theorem-Ideal Gas MixturesDocument24 pagesLecture-5 Gibbs Theorem-Ideal Gas MixturesShiavm PatelNo ratings yet
- MT L23 DistillationDocument8 pagesMT L23 DistillationShiavm PatelNo ratings yet
- MT L 14-17 DistillationDocument25 pagesMT L 14-17 DistillationShiavm PatelNo ratings yet
- Lecture-4 Chemical Potential & Its ApplicationsDocument13 pagesLecture-4 Chemical Potential & Its ApplicationsShiavm PatelNo ratings yet
- Lecture-6 Property Change of Mixing-Ideal SolutionDocument7 pagesLecture-6 Property Change of Mixing-Ideal SolutionShiavm PatelNo ratings yet
- Distillation L6Document21 pagesDistillation L6Shiavm PatelNo ratings yet
- Distillation L7 and 8Document23 pagesDistillation L7 and 8Shiavm PatelNo ratings yet
- Chapter 2 - Assignment 1Document7 pagesChapter 2 - Assignment 1Shiavm PatelNo ratings yet
- Distillation L5Document25 pagesDistillation L5Shiavm PatelNo ratings yet
- Chapter - 2 - Molecular Diffusion - Part-1Document76 pagesChapter - 2 - Molecular Diffusion - Part-1Shiavm PatelNo ratings yet
- MT1 Distillation L3 v1Document23 pagesMT1 Distillation L3 v1Shiavm PatelNo ratings yet
- Examination: Regular Seat No: - Date: 28/11/2020 - Day: - Time Duration: 1.5 Hours Max. Marks: 30Document8 pagesExamination: Regular Seat No: - Date: 28/11/2020 - Day: - Time Duration: 1.5 Hours Max. Marks: 30Shiavm PatelNo ratings yet
- Hydraulic Schematic 6050 FS: Old Hydraulikschaltplan 6050 LSDocument1 pageHydraulic Schematic 6050 FS: Old Hydraulikschaltplan 6050 LSJHONATAN ESTEBAN VALENZUELA ALBIÑONo ratings yet
- Calire Termica RapidaDocument9 pagesCalire Termica RapidaParvan VasiliNo ratings yet
- Solutions Book: 2016 Chemistry Written ExaminationDocument43 pagesSolutions Book: 2016 Chemistry Written Examinationfullname isNo ratings yet
- Fishing Procedure and ToolDocument26 pagesFishing Procedure and ToolWilly Regis SoukilaNo ratings yet
- Megan WolfeDocument8 pagesMegan WolfeAvigyanSinhaNo ratings yet
- 2022 Asme Codes Part1Document1 page2022 Asme Codes Part1Sachin KumarNo ratings yet
- Gromacs 4.5 Manual Beta2Document342 pagesGromacs 4.5 Manual Beta2Aniket MagarkarNo ratings yet
- 1 s2.0 S1359646219300363 MainDocument6 pages1 s2.0 S1359646219300363 Mainswaminathan G.No ratings yet
- Ampere's LawDocument18 pagesAmpere's LawLwazi SiyabongaNo ratings yet
- Exact Mathematical Expressions of The Proton To Electron Mass RatioDocument6 pagesExact Mathematical Expressions of The Proton To Electron Mass RatioStergios PellisNo ratings yet
- RoClean Selection GuideDocument2 pagesRoClean Selection GuideramonmzaNo ratings yet
- Diagnostic Methods of Oil Immersed TransformersDocument13 pagesDiagnostic Methods of Oil Immersed Transformerskrmurali2000100% (1)
- Experiment of Shell & Tube Heat ExchangerDocument29 pagesExperiment of Shell & Tube Heat ExchangerNanaNaurah9578% (9)
- Oenoflow XLDocument6 pagesOenoflow XLAlvaroNo ratings yet
- HIT-RE 100: Safety Information For 2-Component-ProductsDocument23 pagesHIT-RE 100: Safety Information For 2-Component-ProductsLennieCartujanoLuceñoNo ratings yet
- Santhosh P Nano Technology PPT 1Document7 pagesSanthosh P Nano Technology PPT 1ASHWIN MNo ratings yet
- KLM Refining Technical Rev 3Document6 pagesKLM Refining Technical Rev 3Muhammad Abdul RaufNo ratings yet
- HoldernessLambert ANewCertificateChemistry4thEd TextDocument287 pagesHoldernessLambert ANewCertificateChemistry4thEd Textdamijep736No ratings yet
- Diversey - C3010 PDFDocument8 pagesDiversey - C3010 PDFNikesh ShahNo ratings yet
- Molarity, Molality, Normality, and Mass Percent Worksheet II Answer Key 11-12 PDFDocument3 pagesMolarity, Molality, Normality, and Mass Percent Worksheet II Answer Key 11-12 PDFGerald KamulanjeNo ratings yet
- Super ProDocument32 pagesSuper ProguisselaNo ratings yet
- Liquid Diffusion eDocument17 pagesLiquid Diffusion elaoy aolNo ratings yet
- PHY 2 - Problem Solution of CH 1Document10 pagesPHY 2 - Problem Solution of CH 1Mohamed El-GoharyNo ratings yet
- Industrial Scale Production of Palm Super Olein Using Modified and Innovative Dry Fractionation TechniqueDocument12 pagesIndustrial Scale Production of Palm Super Olein Using Modified and Innovative Dry Fractionation TechniqueeyobNo ratings yet
- Sulution Manual - Well Logging For Earth ScientistsDocument15 pagesSulution Manual - Well Logging For Earth Scientistsferhatc560% (2)
- Percdc Cns Geas 1Document9 pagesPercdc Cns Geas 1Charles Adrian CNo ratings yet
- Gr12 MCQDocument87 pagesGr12 MCQkaaviya.mksasiNo ratings yet
- NMR and Transition MetalsDocument21 pagesNMR and Transition MetalskrisnuNo ratings yet
- Brochu Et Al. (1986) - Dispersion of Crude Oil in Seawater, The Role of Synthetic SurfactantsDocument23 pagesBrochu Et Al. (1986) - Dispersion of Crude Oil in Seawater, The Role of Synthetic SurfactantsnimaaandmNo ratings yet
- Collision Theory Is The Key To Understanding Why Some Reactions Are Faster Than OthersDocument16 pagesCollision Theory Is The Key To Understanding Why Some Reactions Are Faster Than OthersRicki HanNo ratings yet