Professional Documents
Culture Documents
Blood Transes
Uploaded by
Alexandra Faith ByunOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Blood Transes
Uploaded by
Alexandra Faith ByunCopyright:
Available Formats
BIOCHEMISTRY
TRANS / [BLOOD] chapter 9
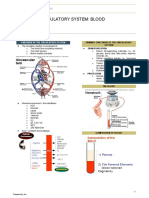
FUNCTIONS OF BLOOD
BLOOD 1. Transport products of digestion
● Aqueous-based solution 2. Transport waste products of
● Plays an essential role in the tissue metabolism
distribution of cells, proteins, 3. Transport oxygen
nutrients, gasses and many other 4. Transport endocrine secretions
biological materials throughout 5. Regulate acid-base balance
organisms. 6. Regulate fluid balance
● Each of us has 5-6 liters of the 7. Regulate the body temperature
complex solution coursing through 8. Protect body against infection
our arteries and veins. 9. Prevent hemorrhage
● Blood may be separated into its two
parts by centrifugation.
● The sediment is composed of three
GENERAL CHARACTERISTICS
types of blood cells: erythrocytes,
leukocytes, and platelets ● Quantity/Volume
(thrombocytes). ○ 6-8% or ⅓ of the total body
● The supernatant, the liquid portion weight or 60-80 milliliter per
called blood plasma, kilogram body weight.
○ A solution consisting of ● Color
approximately 90% water and ○ Oxygenated blood is scarlet
10% dissolved solutes red
■ Such as plasma ○ Deoxygenated blood is dull
proteins, glucose, red
hormones, ● pH
cholesterol, vitamins, ○ 7.35 - 7.45, classified as
inorganic ions, and slightly alkaline
many other ● Specific gravity
biomolecules. ○ 1.060
● The most useful method for
diagnosis of diseases COMPOSITION
○ collection of blood samples
from an individual and clinical 1. Plasma
analysis of changes in the ● Constitutes about 55%
biochemical components ● The blood corpuscles about 45%
● Blood plasma is made up of 90-92%
water and 8-10% solutes, and these
are the following:
BIOCHEMISTRY GIDELINE A. ONDE
a. Plasma proteins ■ Acetone,
■ Serves as nutrition for hydroxybutyric acid
other tissues of the
body. g. Bile acids
■ Contribute to the h. Inorganic constituents
viscosity of the blood. ■ Present in the amount
■ Help in the of 9-11 mg/100 mL of
maintenance of the blood
normal acid-base ■ Ca, Mg, Na, chlorides,
balance. K, phosphates,
■ Provide antibodies sulfates
■ Exert an osmotic
effect i. enzymes
■ Phosphatase,
b. Non-protein nitrogenous amylase, lipase,
substances (NPN transaminase
substances)
■ Intermediary 2. Plasma
metabolites and a. Erythrocytes or red blood
present to the amount corpuscles
of 25-35 mg/100mL of ■ Biconcave disks
blood. devoid of nucleus
■ Include urea, ■ Normally present to
creatinine, uric acid, the amount of
amino acid, ammonia, 4-6M/mm^3
bile pigments. ■ Most important
constituent is
c. Carbohydrates and related hemoglobin,
substances ● Made up of
■ Glucose (80-120 heme and
mg/100 mL of blood) globin; has a
■ Pentose (2-3 normal value
mg/100mL of blood) of 14-16
grams/1oomL
d. Blood lipids of blood.
■ Fats, phospholipids, b. Leukocytes or white blood
cholesterol corpuscles
■ Soldiers of the body
e. immunoglobulins ■ Normally present to
■ Antibodies present in the amount of
blood 4000-11000/cubic and
agranulocytes
f. Ketone bodies
BIOCHEMISTRY GIDELINE A. ONDE
c. Thrombocytes or platelets
BUFFERS IN BLOOD
■ 250000-500000/cubic
mm ● The major buffer system of blood
■ Responsible for blood and other fluids is the carbonic
clotting, which has two acid-bicarbonate conjugate pair.
main steps:
● 1) activation of
prothrombin ● A base added to blood would be
into thrombin neutralize by the following
and reactions:
● 2) conversion
of fibrinogen
into fibrin ● The addition of an acidic
substance to blood also results in
neutralization:
● These reactions illustrate how
blood is protected against pH
changes.
● The actual pH of blood is at the
upper limit of the buffering range
of carbonic acid-bicarbonate and
may not seem as efficient as
desired.
● This inefficiency is remedied by a
reserve supply of gaseous CO2 in
the lungs,
○ Which can replenish
H2CO3 in the blood
by the following
series of equilibrium
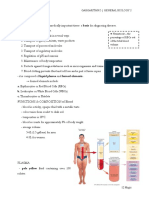
Composition of blood reactions.
● When blood is transferred to
a test tube and is prevented ● The reactions also work in reverse
from clotting, it forms two ● A major product of metabolism, H+,
layers. is removed from cells by the blood
● The transparent yellow top plasma.
layer is plasma, the liquid ● It is neutralized by reaction wtih
portion of blood. carbonate ion and leads to eventual
● The formed elements are in release of CO2(g)
the bottom layer ○ Which is exhaled from the
lungs.
BIOCHEMISTRY GIDELINE A. ONDE
● The carbonic acid-bicarbonate ● Respiratory alkalosis is induced by
conjugate pair is the most important hyperventilation (heavy breathing)
buffer system in biological fluids ○ Which may result from
○ However, it is not the only hysteria, anxiety, or altitude
one. sickness.
● A diverse array of amino acids ,
peptides, and proteins with ionizable
functional groups (-COOH and
ABO BLOOD TYPE
NH3+) assist in buffering.
● A major protein constituent of blood, ● All humans and many other primates
hemoglobin also serves as a can be typed for the ABO blood
buffering agent. group.
● Medical conditions caused by ● There are four principal types: A, B,
changes in blood pH are acidosis AB, and O
and alkalosis. ○ There are two antigens and
● An increas in the [H+] of blood two antibodies that are mostly
(acidosis) may have causes responsible for the ABO
○ That are of metabolic or types.
respiratory origin. ○ The specific combination of
● Metabolic acidosis occurs in these four components
individuals with untreated diabetes determines an individual’s
or in those on starvation diets or on type in most cases.
highprotein, low-fat diets. ● The table below shows the possible
● All of these metabolic conditions permutations of antigens and
lead to ketosis, antibodies with the corresponding
○ The excessive generation of ABO type (“yes” indicates the
ketone bodies, which are presence of a component and “no”
acidic and increase the [H+] indicates its absence in the blood of
of blood. an individual)
● Respiratory acidosis is caused by a
change in [CO2] that is often a
symptom of pulmonary problems
associated with emphysema or
asthma.
○ Untreated acidosis leads to
coma and eventually death.
● An increase in blood pH (alkalosis)
● For example, people with tyoes A
also has metabolic or respiratory
blood will have the A antigen on the
origins.
surface of their red cells.
● Clinical administration of the salts of
○ As a result, anti-A antibodies
metabolic acids (sodium lactate or
will not be produced by them
sodium bicarbonate) in excessive
because they would cause
amounts or cases of severe
the destruction of their own
vomiting cause metabolic alkalosis.
blood.
BIOCHEMISTRY GIDELINE A. ONDE
○ However, if B type blood is
injected into their systems,
anti-B antibodies in their
plasma will recognixe it as
an alien and burst or
agglutinate the introduced
red cells in order to cleanse
the blood of alien protein.
● Individuals with typee O blood do
not produce ABO antigens.
○ Therefore, their blood
normally will not be rejected
when it is given to others
with different ABO types.
○ As a result, type O people
are universal donors for
transfusions, but they can
receive only type O blood
themselves.
○ Those who have type AB
blood do not make any ABO
antibodies.
■ Their blood does not
discriminate against
any other ABO type.
■ Consequently they
are universal
receivers for
transfusions, but their
blood will be
agglutinated when
given to people with
every other type
because they
produce both kinds of
antigens.
BIOCHEMISTRY GIDELINE A. ONDE
You might also like
- Blood TransesDocument5 pagesBlood Transesbaekhyunee exoNo ratings yet
- DR Samar Word Lecture 1 Blood Elalamein For Students 2021-22Document5 pagesDR Samar Word Lecture 1 Blood Elalamein For Students 2021-22AhmedNo ratings yet
- Circulatory System: BloodDocument8 pagesCirculatory System: Bloodino zuii javierNo ratings yet
- Lecture 7a and 7bDocument9 pagesLecture 7a and 7bbuchienjoyerNo ratings yet
- Blood: TransportationDocument29 pagesBlood: TransportationShobhit GajbhiyeNo ratings yet
- Blood Chapter 11Document5 pagesBlood Chapter 11Michoe BanugNo ratings yet
- Physiology and BiochemistryDocument54 pagesPhysiology and BiochemistryDaniNo ratings yet
- Blood Physiology LectureDocument174 pagesBlood Physiology Lectureemmanuelakinola2006No ratings yet
- Week 9 Blood (P) Lecture 1Document18 pagesWeek 9 Blood (P) Lecture 1Fathimath iba AhmedNo ratings yet
- BloodDocument32 pagesBloodPushpa AdhikariNo ratings yet
- Physiology I Blood & Immunity: 1-PlasmaDocument12 pagesPhysiology I Blood & Immunity: 1-Plasmanadine azmyNo ratings yet
- Blood and LymphaticDocument4 pagesBlood and LymphaticCKNo ratings yet
- Anaphy Transes 11.08.23 Circulatory SystemDocument9 pagesAnaphy Transes 11.08.23 Circulatory SystemlumilumiyahNo ratings yet
- Blood Anaphy ReportingDocument52 pagesBlood Anaphy ReportingCza Mae ArsenalNo ratings yet
- BloodDocument127 pagesBloodyapyihao2No ratings yet
- Red Blood Cells, Anemia, and Polycythemia: by DR Shagufta KhaliqDocument67 pagesRed Blood Cells, Anemia, and Polycythemia: by DR Shagufta KhaliqHussi BroNo ratings yet
- Anatomy, Physiology, & Disease: Yna Mae de LeonDocument8 pagesAnatomy, Physiology, & Disease: Yna Mae de LeonYna Mae C. De LeonNo ratings yet
- MS - HemaDocument23 pagesMS - Hemajohn denver floresNo ratings yet
- M11 HAPP LecDocument9 pagesM11 HAPP LecM SNo ratings yet
- Endocrine System Module: Biochemistry and Molecular BiologyDocument179 pagesEndocrine System Module: Biochemistry and Molecular BiologyƯớc Của BếttingNo ratings yet
- L - 28-Composition and Function of Blood. ( (L32) )Document28 pagesL - 28-Composition and Function of Blood. ( (L32) )Turky TurkyNo ratings yet
- Week 14 Blood and Hematopoeitic SystemDocument50 pagesWeek 14 Blood and Hematopoeitic SystemKate Dela cruzNo ratings yet
- Rate of Reticulocyte Release Rate of Removal of Spent Rbcs by Spleen & LiverDocument4 pagesRate of Reticulocyte Release Rate of Removal of Spent Rbcs by Spleen & Liverraiansuyu2495gmail.comNo ratings yet
- BloodDocument73 pagesBloodFedelyn Mae AcaylarNo ratings yet
- CBC Reviewer Anaphy LabDocument9 pagesCBC Reviewer Anaphy LabARVINE JUSTINE CORPUZNo ratings yet
- BloodDocument103 pagesBloodrizwanbas50% (2)
- Anaphy Chapter 19 Blood Doran Mls1 FDocument12 pagesAnaphy Chapter 19 Blood Doran Mls1 FayenaNo ratings yet
- Body FluidsDocument51 pagesBody FluidsCor GuerreroNo ratings yet
- BLOODDocument175 pagesBLOODJohn JimsNo ratings yet
- Blood Lectures 2014 PIO 205Document84 pagesBlood Lectures 2014 PIO 205Philip Abayomi VincentNo ratings yet
- Blood - NotesDocument5 pagesBlood - NotesJenn Mamar CabayaoNo ratings yet
- Cardiovascular System - BloodDocument8 pagesCardiovascular System - BloodVernice LopezNo ratings yet
- Z-05 Digest Part EVDocument49 pagesZ-05 Digest Part EVXaveer AzadNo ratings yet
- Red Blood Cells "Best Module Ever"Document4 pagesRed Blood Cells "Best Module Ever"Jose Emmanuel Dolor100% (1)
- Composition of Blood PlasmaDocument27 pagesComposition of Blood PlasmaSUNIL KUMAR100% (1)
- The BloodDocument24 pagesThe BloodKicki AnderssonNo ratings yet
- Blood Components Where Do They Come From?: Introduction To HaematologyDocument11 pagesBlood Components Where Do They Come From?: Introduction To Haematologydorsa koraeiNo ratings yet
- Physiology of Blood Lectures - DMKDocument197 pagesPhysiology of Blood Lectures - DMKFreelance LeagueNo ratings yet
- Rest of No 1Document16 pagesRest of No 1eman el saeedNo ratings yet
- DR Otodo BloodDocument19 pagesDR Otodo BloodOluwapelumi DimejiNo ratings yet
- 8 Blood ChemistryDocument10 pages8 Blood ChemistryAlexandra DalisayNo ratings yet
- Blood: A. Erythrocytes or Red Blood Cells (RBCS) B. Leukocytes or White Blood Cells (WBCS) C. Thrombocytes or PlateletsDocument10 pagesBlood: A. Erythrocytes or Red Blood Cells (RBCS) B. Leukocytes or White Blood Cells (WBCS) C. Thrombocytes or PlateletsVictor HechanovaNo ratings yet
- 1-Basics in HaematologyDocument19 pages1-Basics in HaematologyWasana MendisNo ratings yet
- Physiology NotesDocument769 pagesPhysiology NotesKishore SenthilkumarNo ratings yet
- Veterinary Physiology BLOODDocument50 pagesVeterinary Physiology BLOODGagan shri Bankira roll-43No ratings yet
- Chapter Five: Blood PhysiologyDocument91 pagesChapter Five: Blood PhysiologyjarssooNo ratings yet
- Composition and FunctionsDocument13 pagesComposition and Functionsarun231187No ratings yet
- Blood Chapter 11Document4 pagesBlood Chapter 11James CastroNo ratings yet
- BloodDocument4 pagesBloodA R F I J U LNo ratings yet
- Lecture 9 Physiology of BloodDocument125 pagesLecture 9 Physiology of BloodAhmed MohammedNo ratings yet
- Blood PhysiologyDocument70 pagesBlood PhysiologySultan AhimedNo ratings yet
- Adobe Scan 12 Feb 2023Document24 pagesAdobe Scan 12 Feb 2023Atul TripathiNo ratings yet
- HSSRPTR - 07-Body Fluids and CirculationDocument15 pagesHSSRPTR - 07-Body Fluids and CirculationUnniNo ratings yet
- 1.07 - The BloodDocument5 pages1.07 - The Blood13PLAN, SENTH RUEN, ANo ratings yet
- Physiology of HematologyDocument114 pagesPhysiology of Hematologyrizki syahrifaNo ratings yet
- Hema Lec Cover2coverDocument61 pagesHema Lec Cover2coverPrincheska TallaNo ratings yet
- KRV, PLAZMA, HEMATOPOEZADocument35 pagesKRV, PLAZMA, HEMATOPOEZASinisa RisticNo ratings yet
- The River of Life Blood Chapter 17Document31 pagesThe River of Life Blood Chapter 17Harshil PatelNo ratings yet
- MD-5 Plasma Cont. Blood CellssDocument20 pagesMD-5 Plasma Cont. Blood CellssvincenzoNo ratings yet
- PEE Module 2 Chapter 5Document17 pagesPEE Module 2 Chapter 5Mardelyn BaynoNo ratings yet
- st15122 PDFDocument10 pagesst15122 PDFJose María Ureta UrzúaNo ratings yet
- 2018 Mark Scheme Final VersionDocument13 pages2018 Mark Scheme Final VersionJackieWilsonNo ratings yet
- chm510 Experiment 3Document7 pageschm510 Experiment 3Naz Helmi100% (1)
- 1152 Lab CarbohydratesDocument8 pages1152 Lab Carbohydratesanyss_afiezaNo ratings yet
- Dental Ceramics Used in DentistryDocument10 pagesDental Ceramics Used in DentistryERIKA BLANQUETNo ratings yet
- IRP 1dandfblocksDocument21 pagesIRP 1dandfblocksDhrutvan Reddy ReddiwaryNo ratings yet
- A Comparative Chemical Analytical Study of Mercuric Chloride (Veeram) On Before and After PurificationDocument7 pagesA Comparative Chemical Analytical Study of Mercuric Chloride (Veeram) On Before and After Purificationjmanuel108yahoo.co.ukNo ratings yet
- 2008-6-13 - What Are Your Pipeline Integrity Key Performance IndicatorsDocument62 pages2008-6-13 - What Are Your Pipeline Integrity Key Performance IndicatorsSate Joglo TresnoNo ratings yet
- The Nitrogen CycleDocument3 pagesThe Nitrogen CycleBon Joey J. BernestoNo ratings yet
- H2 - Prelim 2009 Paper1Document16 pagesH2 - Prelim 2009 Paper1Augustine NgNo ratings yet
- Jce 2007 P 0124 WDocument25 pagesJce 2007 P 0124 WAlexaNo ratings yet
- Alteration and Fluid Inclusion (Arif M)Document52 pagesAlteration and Fluid Inclusion (Arif M)Akma Kamarudin100% (1)
- Iso 13507 2012Document9 pagesIso 13507 2012Logan KNo ratings yet
- Yellow Oxide 42Document1 pageYellow Oxide 42ahboon145No ratings yet
- Amino Acids Reference Chart Sigma AldrichDocument3 pagesAmino Acids Reference Chart Sigma AldrichcfmonarquiaNo ratings yet
- Lecture 1 - Milk CompositionDocument46 pagesLecture 1 - Milk CompositionAmirul AimanNo ratings yet
- Properties and Characterization of A Clay Raw Material From Miličinica (Serbia) For Use in The Ceramic IndustryDocument13 pagesProperties and Characterization of A Clay Raw Material From Miličinica (Serbia) For Use in The Ceramic IndustryFOUTOUNo ratings yet
- Chemicals Zetag DATA Powder Zetag 4105 - 1110Document2 pagesChemicals Zetag DATA Powder Zetag 4105 - 1110PromagEnviro.comNo ratings yet
- Dosage Forms Module 1Document2 pagesDosage Forms Module 1Lyka TamarayNo ratings yet
- Aspirin Synthesis Post LabDocument3 pagesAspirin Synthesis Post LabMiracle VerteraNo ratings yet
- CH 07 Reactive DyesDocument15 pagesCH 07 Reactive DyesNabarupa BoseNo ratings yet
- Oxygen Family - Theory Notes With Illustrative Examples (Unlocked by WWW - Freemypdf.com)Document15 pagesOxygen Family - Theory Notes With Illustrative Examples (Unlocked by WWW - Freemypdf.com)Imran Khan100% (2)
- Exp 9Document10 pagesExp 9June Angela BacayNo ratings yet
- Heterocyclic Compounds 3 فصل ثاني مرحلة ثانية مادة العضويةDocument59 pagesHeterocyclic Compounds 3 فصل ثاني مرحلة ثانية مادة العضويةMahendra ChoudharyNo ratings yet
- Worksheet On General ChemistryDocument2 pagesWorksheet On General ChemistryMay Conde Aguilar50% (2)
- Lesson Plan 12 Redox ReactionDocument9 pagesLesson Plan 12 Redox Reactionnur rizkhana harianiNo ratings yet
- Conc - Tech Lec-1-1Document63 pagesConc - Tech Lec-1-1TEWODROS TADDESENo ratings yet
- Chemistry AS 2020 Flashcards - QuizletDocument3 pagesChemistry AS 2020 Flashcards - QuizletyousafNo ratings yet
- As Chemistry Answer BookDocument30 pagesAs Chemistry Answer Booksaviochow80% (5)
- ICH Quality Guidelines: An Implementation GuideFrom EverandICH Quality Guidelines: An Implementation GuideAndrew TeasdaleNo ratings yet
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- It's Elemental: The Hidden Chemistry in EverythingFrom EverandIt's Elemental: The Hidden Chemistry in EverythingRating: 4 out of 5 stars4/5 (10)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeFrom EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeRating: 5 out of 5 stars5/5 (1)
- Guidelines for Defining Process Safety Competency RequirementsFrom EverandGuidelines for Defining Process Safety Competency RequirementsRating: 3 out of 5 stars3/5 (1)
- The Production of Volatile Oils and Perfumery Plants in the United StatesFrom EverandThe Production of Volatile Oils and Perfumery Plants in the United StatesNo ratings yet
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeFrom EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeNo ratings yet
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Taste: Surprising Stories and Science About Why Food Tastes GoodFrom EverandTaste: Surprising Stories and Science About Why Food Tastes GoodRating: 3 out of 5 stars3/5 (20)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (1)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- The Billion-Dollar Molecule: The Quest for the Perfect DrugFrom EverandThe Billion-Dollar Molecule: The Quest for the Perfect DrugRating: 5 out of 5 stars5/5 (2)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- Essential Chemistry for Formulators of Semisolid and Liquid DosagesFrom EverandEssential Chemistry for Formulators of Semisolid and Liquid DosagesRating: 5 out of 5 stars5/5 (2)