Professional Documents
Culture Documents
Rate of Reaction Lab (Magnesium Ribbon)
Uploaded by
Naldo 64Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Rate of Reaction Lab (Magnesium Ribbon)
Uploaded by
Naldo 64Copyright:
Available Formats
TITLE: Rates of Reactions
AIM: To investigate the effect of concentration on the rate of reaction
APPARATUS & MATERIALS:
Magnesium ribbon
Sand (emery) paper
Beakers
Stop clock
Distilled water
Hydrochloric acid
Cut five 3 cm lengths of clean magnesium ribbon (clean with emery paper until shiny if necessary). Pour 50 cm3
of the 2M acid provided, into the beaker, and add one piece of the ribbon. At the same time start the stop-clock.
Note the time taken for the ribbon to disappear completely.
Repeat the procedure with the four remaining pieces of ribbon, adding 40, 30, 20, 10 cm3 of acid respectively,
and make up each solution to 50 cm3, by adding distilled water, before adding the ribbon in each case. Record
the various times and tabulate your results.
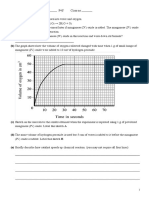
(i) Plot a graph of volume of acid used against the time taken for complete dissolution. Plot a second
graph against reciprocal of time (1/T).
(ii) What can you say about the rate of the reaction, with respect to the concentration of the acid, from
your graphs.
(iii) If the beakers were shaken during the reaction, would this have affected the reaction rate? Explain
your answer.
(iv) (a) Why was it necessary to clean the magnesium ribbon to study this particular reaction?
(b) Why was it unnecessary to convert the volume of acid used to molarities, in order to plot the
graph?
(v) (a) What are some sources of error in this experiment?
(b) What factors are constant in this experiment?
Volume of acid (cm3) Volume of water (cm3) Time (sec) 1
(sec-1)
Time
50 0
40 10
30 20
20 30
10 40
You might also like
- National 5 Exam Style QuestionsDocument24 pagesNational 5 Exam Style Questionsmilneda31No ratings yet
- Rates of Reaction - Disappearing Cross LabDocument3 pagesRates of Reaction - Disappearing Cross Lab4L Anisha SieudassNo ratings yet
- Past Paper Questions Uncert in Meas and TitrimetryDocument3 pagesPast Paper Questions Uncert in Meas and TitrimetryLisa SawhNo ratings yet
- Rate Reaction Lloaana 2020Document8 pagesRate Reaction Lloaana 2020Lloaana 12No ratings yet
- Experimen T Number of Acid/ CM Volume of Thiosulphate/cm Volume of Water/cm Time (T) /s 1 (S) TDocument2 pagesExperimen T Number of Acid/ CM Volume of Thiosulphate/cm Volume of Water/cm Time (T) /s 1 (S) TcrissaniaNo ratings yet
- Experiment 2: Rate of Reaction and Initial Rates: ObjectiveDocument5 pagesExperiment 2: Rate of Reaction and Initial Rates: ObjectiveSiti SafarNo ratings yet
- Chapter 4 SepticTank Design and Construction by Haile.BDocument14 pagesChapter 4 SepticTank Design and Construction by Haile.BANDLENATUNo ratings yet
- Rates of ReactionDocument4 pagesRates of Reactionrussellivans12No ratings yet
- Biology PND EnzymeDocument4 pagesBiology PND EnzymeVishmeta SeenarineNo ratings yet
- You Will Learn About:: - Definition Rate of ReactionDocument39 pagesYou Will Learn About:: - Definition Rate of ReactionZam EjamNo ratings yet
- Exercise On Rate of ReactioDocument4 pagesExercise On Rate of ReactiohahaNo ratings yet
- 01 Kadar Tindak Balas Soalan StrukturDocument7 pages01 Kadar Tindak Balas Soalan StrukturAzalida Md YusofNo ratings yet
- Assumption English School: Science DepartmentDocument3 pagesAssumption English School: Science DepartmentTimothy HandokoNo ratings yet
- Analytical Qns 2020 BioDocument9 pagesAnalytical Qns 2020 BioJacinta HerewithNo ratings yet
- 1 Diffusion Brownian Motion Solids Liquids Gases QP CIE IGCSE Chemistry Practical UpdatedDocument7 pages1 Diffusion Brownian Motion Solids Liquids Gases QP CIE IGCSE Chemistry Practical UpdatedPamela PadillaNo ratings yet
- Rate of Reaction - CatalystDocument19 pagesRate of Reaction - CatalystSAGUNTHALA MANIAM -No ratings yet
- Effect of Catalyst On The Rate of Reaction Teacher's GuideDocument8 pagesEffect of Catalyst On The Rate of Reaction Teacher's GuideFia RafiahNo ratings yet
- Determination of Reaction Rate ConstantDocument7 pagesDetermination of Reaction Rate ConstantJoyce VicenteNo ratings yet
- Suggested Answers Ting.5Document28 pagesSuggested Answers Ting.5engNo ratings yet
- Chemistry P3 0003Document9 pagesChemistry P3 0003Karoki Francis KagombeNo ratings yet
- Leonardo Lezama0530 - Concentration Vs Rate - Lab Write UpDocument11 pagesLeonardo Lezama0530 - Concentration Vs Rate - Lab Write Upleonardo lezama0431No ratings yet
- Chemistry SPM Potential Questions-Form5chap1 2Document15 pagesChemistry SPM Potential Questions-Form5chap1 2EloiseCalaisNo ratings yet
- Chemistry 3A - Special Schools Syndicate Joint Examination F6 2023Document4 pagesChemistry 3A - Special Schools Syndicate Joint Examination F6 2023kulwayohana61No ratings yet
- Ease 4 Chemistry Grade 8 19-20Document8 pagesEase 4 Chemistry Grade 8 19-20fajarNo ratings yet
- Campion College: WorksheetDocument3 pagesCampion College: WorksheetMaliq MorrisNo ratings yet
- Sem 2 2011-2012 (June) SAB 2912 SET ADocument6 pagesSem 2 2011-2012 (June) SAB 2912 SET AAfendi AriffNo ratings yet
- CEng2142 REG 2011 Test1 Examination Paper SetDocument10 pagesCEng2142 REG 2011 Test1 Examination Paper SetCaalchisaa MargaaNo ratings yet
- Discussion Batch SaponificationDocument4 pagesDiscussion Batch SaponificationSuria Seri SulyanaNo ratings yet
- 21B2043 Rusydi SC2243 Exp2Document7 pages21B2043 Rusydi SC2243 Exp2raniyaNo ratings yet
- Fin 00253Document10 pagesFin 00253ayat arahmanNo ratings yet
- Module 62 Rate of Reaction Concentration Effect - DwiDocument2 pagesModule 62 Rate of Reaction Concentration Effect - Dwirudi_zNo ratings yet
- Co4: Effect of Concentration On Rate of Reaction: ObjectivesDocument2 pagesCo4: Effect of Concentration On Rate of Reaction: ObjectivesSebastian Genesis ViduyaNo ratings yet
- CHEMISTRYset 1 P3Document7 pagesCHEMISTRYset 1 P3Yu YanNo ratings yet
- 7.6 Quantitative Analysis Assignment 2 AnswerDocument1 page7.6 Quantitative Analysis Assignment 2 AnswerTaryl ThomasNo ratings yet
- Jomo Kenyatta University OF Agriculture and Technology University Examinations 2014/2015Document4 pagesJomo Kenyatta University OF Agriculture and Technology University Examinations 2014/2015Joseph NjugunaNo ratings yet
- Unit 4 Review Reaction Rates Answers To ReviewDocument8 pagesUnit 4 Review Reaction Rates Answers To ReviewANGELYN SANTOSNo ratings yet
- Volumetric Analysis QuestionsDocument3 pagesVolumetric Analysis QuestionsJake Robinson100% (8)
- Chem - Rates of Reaction ProblemsDocument6 pagesChem - Rates of Reaction ProblemslyagobaNo ratings yet
- Chemical KineticsDocument5 pagesChemical KineticsTimothy HandokoNo ratings yet
- Y09 TestDocument2 pagesY09 TestKissiedu YirenkyiNo ratings yet
- Rate of Reaction of Sodium Thiosulfate and Hydrochloric AcidDocument5 pagesRate of Reaction of Sodium Thiosulfate and Hydrochloric AcidTeacher AlexNo ratings yet
- Quiz 1 With AnswerDocument3 pagesQuiz 1 With AnswerAltra ZNo ratings yet
- TCW3204201505 Irrigation Systems DesignDocument6 pagesTCW3204201505 Irrigation Systems DesignNyashah FelixNo ratings yet
- Experiment 1 Lab ReportDocument7 pagesExperiment 1 Lab ReportChaapi KimNo ratings yet
- Rate of Reaction f5 (Worksheet)Document35 pagesRate of Reaction f5 (Worksheet)Derek Ma67% (3)
- Titration CalculationsDocument21 pagesTitration CalculationsG M Ali KawsarNo ratings yet
- DC91860 PDFDocument4 pagesDC91860 PDFavocado rollNo ratings yet
- CIE472 Hydrology Mock Test 2020Document4 pagesCIE472 Hydrology Mock Test 2020Chris KapendaNo ratings yet
- Rate of Reaction Part 1Document3 pagesRate of Reaction Part 1Subesh ShanmugamNo ratings yet
- MSC Exam Practice QuestionsDocument3 pagesMSC Exam Practice QuestionsShahzaib MalikNo ratings yet
- CGP Chemistry L2 Mark SchemeDocument2 pagesCGP Chemistry L2 Mark SchemeBeverleyNo ratings yet
- (M1.2) REVIEW & COMPLETE - Practical 4.1 The Kinetics of The Reaction Between CaCO3 and HCLDocument3 pages(M1.2) REVIEW & COMPLETE - Practical 4.1 The Kinetics of The Reaction Between CaCO3 and HCLSalma ShakiraNo ratings yet
- Rates of ReactionDocument77 pagesRates of ReactionFatema KhatunNo ratings yet
- Pastpapers 4.1Document70 pagesPastpapers 4.1Benson Mwathi Mungai100% (2)
- RKDocument6 pagesRKKou UrakiNo ratings yet
- Lab Report Exp 3 Che 142 Group 5 1eDocument7 pagesLab Report Exp 3 Che 142 Group 5 1eNUR QURRATU AINI WEHAIZEDNo ratings yet
- Ester HydrolysisDocument3 pagesEster Hydrolysissheik abdullaNo ratings yet
- Esterification of Ethanol in A Batch Reactor in Presence of H2SO4Document17 pagesEsterification of Ethanol in A Batch Reactor in Presence of H2SO4MD.Khairul EducationNo ratings yet