Professional Documents
Culture Documents
Organic Name Reactions
Uploaded by
Pratham ZalaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Organic Name Reactions
Uploaded by
Pratham ZalaCopyright:
Available Formats
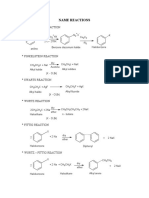
ORGANIC NAME REACTION
1. Finkelstein
CH3Br + NaI CH3-I + NaBr
Swarts CH3Br + AgF CH3F + AgBr
2.
CH3
Friedel-
Anhydrous AlCl3
Crafts
+ H 3C Cl
Alkylation
3.
COCH 3
Friedel-
CH 3COCl
Crafts
4. Acylation
Anhydrous AlCl3
2Na
Wurtz H 3C Cl + Cl CH3 H 3C CH3 + Na Cl
5.
Cl Cl
Fittig +
2Na
+ Na Cl
6. Dry ether
Cl
Wurtz-Fittig 2Na
+ Cl CH3 CH3 + Na Cl
Dry ether
7.
OH OH
ON a
Na OH i) CO2 COOH
Kolbe
ii) H+
8.
Reimer-
Tiemann
9.
10. Williamson CH3-Br + CH3-ONa CH3-O- CH3 + NaBr
H3O+
Stephen H 3C CN + SnCl2 + HCl H 3C CH NH H 3C CHO
11.
CH3 CHO
CrO2Cl2
Etard
H3O+
12.
CHO
Gatterman – CO / HCl
Koch
Anhydrous AlCl3
13.
Lokesh Suredia K V ONGC MEHSANA Page 1
ORGANIC NAME REACTION
O H2 O
Rosenmund
C C
reduction H 3C Cl H 3C H
Pd / BaSO 4
14.
O
Clemmensen Zn - Hg
C H 3C CH2 CH3
reduction Conc. HCl
15. H 3C CH3
O
Wolff-Kishner i) NH2-NH2
reduction C H 3C CH2 CH3
16. H3C CH3 ii) KOH / Ethylene glycol /
17. Tollens’ test R-CHO + 2 [Ag(NH3)2]+ + 3 OH- R-COO- + 2Ag + 2H2O + 4 NH3
18. Fehling’s test R-CHO + 2 Cu 2+
+ 5 OH -
R-COO + Cu2O-
+ 3H2O
O
I2 / NaOH
Iodoform C CHI3 + CH3COONa
19. H 3C CH3 OR, NaOI
OH
dil NaOH
Aldol H3C CH CH2 CHO H3C CH CH
2 H 3C CHO
condensation
20.
Conc. NaOH
21.
Cannizzaro HCHO
+ HCHO HCOON a + H 3C OH
i) Cl2 / Red Phosphorus
Hell-Volhard- H 3C COOH H 2C COOH
Zelinsky (HVZ)
ii) H2O
22. Cl
O
Hoffmann Br2
bromamide H 3C C NH2 H3C NH2
23. degradation NaOH
Carbylamine
24. R-NH2 + CHCl3 + 3 KOH R-NC + 3 KCl + 3 H2O
NH2 + -
N 2 Cl
NaNO 2 + dil HCl
Diazotisation
273 - 278 K
25.
+ -
N 2 Cl Cl
Sandmeyer. CuCl / HCl
N2
+
26.
+ -
N 2 Cl Cl
Gatterman Cu / HCl
N2
+
27.
+ -
OH-
Coupling N 2 Cl + H OH N N OH
28.
Lokesh Suredia K V ONGC MEHSANA Page 2
You might also like
- Organic Chemistry Reacions SummaryDocument22 pagesOrganic Chemistry Reacions SummaryvgettinfatNo ratings yet
- ENVIRONMENTAL HEALTH (Compiled) PDFDocument119 pagesENVIRONMENTAL HEALTH (Compiled) PDFHarlyn PajonillaNo ratings yet
- Organic Chemistry ChartsDocument84 pagesOrganic Chemistry ChartsPRIYANSHU KUMARNo ratings yet
- Organic Chemistry ChartsDocument84 pagesOrganic Chemistry ChartsHarish100% (2)
- Organic Chemistry ChartsDocument84 pagesOrganic Chemistry ChartsPRIYANSHU KUMARNo ratings yet
- Sand Compaction MethodDocument124 pagesSand Compaction Methodisaych33ze100% (1)
- Reaction of Ketone CompleteDocument1 pageReaction of Ketone CompleteJoko SusiloNo ratings yet
- Ip 19 3RD EditionDocument240 pagesIp 19 3RD EditionSumanta Bhaya100% (13)
- Amazon Invoice Books 4Document1 pageAmazon Invoice Books 4raghuveer9303No ratings yet
- Active and Passive Voice quizDocument2 pagesActive and Passive Voice quizM3xobNo ratings yet
- Tabla de Valores de Pka: S. Ege "Química Orgánica" Ed Reverté S.A. 1997Document7 pagesTabla de Valores de Pka: S. Ege "Química Orgánica" Ed Reverté S.A. 1997loaca95No ratings yet
- Organic Name ReactionsDocument2 pagesOrganic Name ReactionsShruti MohrilNo ratings yet
- All Named ReactionsDocument3 pagesAll Named ReactionsSamrathsingh Hayer100% (1)
- Carbonyl Compounds and Carboxylic Acid - Med Easy - Yakeen 2.0 2024 (Legend)Document12 pagesCarbonyl Compounds and Carboxylic Acid - Med Easy - Yakeen 2.0 2024 (Legend)agrawaltwinkle2005No ratings yet
- Short Notes by SK SirDocument8 pagesShort Notes by SK SirJay MeenaNo ratings yet
- MINDMAP Alkene, Benzene, HaloalkaneDocument3 pagesMINDMAP Alkene, Benzene, HaloalkaneLeow JiashengNo ratings yet
- Organic TuteDocument1 pageOrganic TuteDimuthu SandaruwanNo ratings yet
- Recall Alkyl Halides (Haloalkanes) : The Polarity and Strength of The C-X BondDocument32 pagesRecall Alkyl Halides (Haloalkanes) : The Polarity and Strength of The C-X BondafafNo ratings yet
- GOC Class11thDocument38 pagesGOC Class11thAnju SehrawatNo ratings yet
- Alcohol, Phenols and Ethers Ch-10Document19 pagesAlcohol, Phenols and Ethers Ch-10Literal ShTNo ratings yet
- Cbse Test Paper-03 CLASS - XII CHEMISTRY (Aldehydes, Ketones and Carboxylic Acids) (Answer) Topic:-ConversionsDocument2 pagesCbse Test Paper-03 CLASS - XII CHEMISTRY (Aldehydes, Ketones and Carboxylic Acids) (Answer) Topic:-ConversionsShreyash KolekarNo ratings yet
- Chloroform - Memory Map: ReactionsDocument1 pageChloroform - Memory Map: ReactionsAryan GuptaNo ratings yet
- Electrophilic Aromatic SubstitutionDocument5 pagesElectrophilic Aromatic Substitutioneman mamdohNo ratings yet
- FREE DOWNLOAD Guide to Alcohols, Phenols and EthersDocument19 pagesFREE DOWNLOAD Guide to Alcohols, Phenols and EthersParam MNo ratings yet
- Practice Exercise C17Document5 pagesPractice Exercise C17utpNo ratings yet
- Super Important Reaction (Repaired)Document5 pagesSuper Important Reaction (Repaired)Madhav BhatiNo ratings yet
- Super Important Reaction (Repaired)Document5 pagesSuper Important Reaction (Repaired)Rudra PratapNo ratings yet
- Super Important Reaction (Repaired)Document5 pagesSuper Important Reaction (Repaired)AlphaNo ratings yet
- Uace Chem Guide To Mechanism and SynthesisDocument60 pagesUace Chem Guide To Mechanism and SynthesisNelima Stella mercy100% (1)
- Uace Chem Guide To Mechanism and SynthesisDocument60 pagesUace Chem Guide To Mechanism and SynthesisNelima Stella mercyNo ratings yet
- Organic Chemistry Oxidation ReactionsDocument9 pagesOrganic Chemistry Oxidation Reactionsgamer boomerNo ratings yet
- TutorialDocument27 pagesTutorialSiti NuraqidahNo ratings yet
- +2 NEET IntelliQuest PCB-3 (28.01.2021) AnsKey - 10645292Document3 pages+2 NEET IntelliQuest PCB-3 (28.01.2021) AnsKey - 10645292swap2005sharmaNo ratings yet
- Organic Chemistry II Problem Set Reaction of Substituted BenzeneDocument4 pagesOrganic Chemistry II Problem Set Reaction of Substituted BenzenesaddamixoNo ratings yet
- Obtención de Alcoholes por Sustitución Nucleófila y Reactivos de GrignardDocument7 pagesObtención de Alcoholes por Sustitución Nucleófila y Reactivos de GrignardMARITZA QUISPE VILLARREALNo ratings yet
- Dhoom # 9 Haloalkane & Haloarene in One Shot (10.6.2020)Document156 pagesDhoom # 9 Haloalkane & Haloarene in One Shot (10.6.2020)Jeet RathodNo ratings yet
- Important Name Reactions by Vineet Khatri SirDocument4 pagesImportant Name Reactions by Vineet Khatri SirVishalNo ratings yet
- Matriculation Chemistry (Amino Acids) Part 2Document10 pagesMatriculation Chemistry (Amino Acids) Part 2ridwanNo ratings yet
- Alkyl HalidesDocument26 pagesAlkyl Halidesharerambaghel906No ratings yet
- ALKANESREACTIONSDocument5 pagesALKANESREACTIONSSana ImamNo ratings yet
- Presentation Alkyl HalidesDocument20 pagesPresentation Alkyl HalidesCaroline MuthoniNo ratings yet
- 12 Chemistry Haloalkanes and Haloarenes Test 05 Answer s2l6 PDFDocument2 pages12 Chemistry Haloalkanes and Haloarenes Test 05 Answer s2l6 PDFShreyash KolekarNo ratings yet
- Haloalkanes and Haloarenes1Document15 pagesHaloalkanes and Haloarenes1Poorni RenuNo ratings yet
- Aldehydes, Ketones and Carboxylic Acids ChapterDocument1 pageAldehydes, Ketones and Carboxylic Acids ChapterRishi KeshNo ratings yet
- Name Reactions: Sandmeyer'S ReactionDocument9 pagesName Reactions: Sandmeyer'S ReactionSai Krishnan100% (1)
- Reaction of KetonesDocument1 pageReaction of KetonesJoko SusiloNo ratings yet
- Flow Chart - HydrocarbonsDocument77 pagesFlow Chart - HydrocarbonsKalyan Reddt100% (2)
- s19 Ac Quim 10 Viellard Esteban 2Document1 pages19 Ac Quim 10 Viellard Esteban 2Esteban Mauricio Viellard CorralesNo ratings yet
- Hydrocarbon To Amines Short NotesDocument31 pagesHydrocarbon To Amines Short NotesMd AmanNo ratings yet
- ABC 3 (Theory Exercise)Document11 pagesABC 3 (Theory Exercise)Mayank GoyalNo ratings yet
- Aldehidos y Cetonas 2022 IiDocument5 pagesAldehidos y Cetonas 2022 IiJannina Carbajal VelásquezNo ratings yet
- Carboxylic Acid & Acid Derivatives and Amines: Exercise - IDocument3 pagesCarboxylic Acid & Acid Derivatives and Amines: Exercise - ILifestyle BoomNo ratings yet
- Vollhardt Chapter 18 OChem PracticeDocument23 pagesVollhardt Chapter 18 OChem PracticeDanNo ratings yet
- 12 Aldehydes Ketones and Carboxylic AcidsDocument2 pages12 Aldehydes Ketones and Carboxylic AcidsPrasannaNo ratings yet
- Solution of Chemistry HSSC-II (3rd Set)Document11 pagesSolution of Chemistry HSSC-II (3rd Set)Ujala ShahidNo ratings yet
- ETHERS: A GUIDE TO PROPERTIES AND PREPARATION METHODSDocument12 pagesETHERS: A GUIDE TO PROPERTIES AND PREPARATION METHODSkumar swamyNo ratings yet
- C - Sol - Ch-26 - Aldehydes Ketones and Carboxylic AcidsDocument19 pagesC - Sol - Ch-26 - Aldehydes Ketones and Carboxylic AcidsHimanshi ChahalNo ratings yet
- Sample MCQ Organic Chemistry Sem II PSCH203 BacklogDocument4 pagesSample MCQ Organic Chemistry Sem II PSCH203 BacklogganesanneelamuruganNo ratings yet
- Cpp-Alkyl HalideDocument5 pagesCpp-Alkyl Halidekrishnagamer565No ratings yet
- CLS ENG 22 23 XII Che Target 5 Level 1 Chapter 13Document62 pagesCLS ENG 22 23 XII Che Target 5 Level 1 Chapter 13Harsh JakharNo ratings yet
- Organic Chemistry ChartsDocument84 pagesOrganic Chemistry ChartsPRIYANSHU KUMARNo ratings yet
- CBSE-Mock-Test - Part-Test-2 - C-XII - HInts & Sols - Chemistry - 10-3-22Document3 pagesCBSE-Mock-Test - Part-Test-2 - C-XII - HInts & Sols - Chemistry - 10-3-22Swastik PandeyNo ratings yet
- Chemical Vapor DepositionDocument20 pagesChemical Vapor DepositionYong Jae KwonNo ratings yet
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- MS PB-1 Set 1 (A) Maths XiiDocument8 pagesMS PB-1 Set 1 (A) Maths XiiPratham ZalaNo ratings yet
- The Metabolic Map Carbohydrates AtfDocument3 pagesThe Metabolic Map Carbohydrates AtfJoax Wayne SanchezNo ratings yet
- DocumentDocument2 pagesDocumentPratham ZalaNo ratings yet
- Daily Test Planner 2022-23Document1 pageDaily Test Planner 2022-23Pratham ZalaNo ratings yet
- Tips For ConversionDocument2 pagesTips For ConversionPratham ZalaNo ratings yet
- Flash Cards For Bio PracticalsDocument4 pagesFlash Cards For Bio PracticalsPratham ZalaNo ratings yet
- Determination of Population Density and Percentage Frequency by Quadrat Method - Solving The Problems - Botany PracticalsDocument7 pagesDetermination of Population Density and Percentage Frequency by Quadrat Method - Solving The Problems - Botany PracticalsPratham ZalaNo ratings yet
- Test Series-Try TheseDocument35 pagesTest Series-Try ThesePratham ZalaNo ratings yet
- Capsule For Low AchieversDocument17 pagesCapsule For Low AchieversPratham Zala100% (1)
- Organic ExplanationsDocument4 pagesOrganic ExplanationsManjunath NaikNo ratings yet
- Project Certificate - ACCDocument5 pagesProject Certificate - ACCPratham ZalaNo ratings yet
- Pollination Methods and MechanismsDocument5 pagesPollination Methods and MechanismsPratham ZalaNo ratings yet
- Mark Wildon - Representation Theory of The Symmetric Group (Lecture Notes) (2015)Document34 pagesMark Wildon - Representation Theory of The Symmetric Group (Lecture Notes) (2015)Satyam Agrahari0% (1)
- 2 Both Texts, and Then Answer Question 1 On The Question Paper. Text A: Esports in The Olympic Games?Document2 pages2 Both Texts, and Then Answer Question 1 On The Question Paper. Text A: Esports in The Olympic Games?...No ratings yet
- Taylor Introms11GE PPT 03Document40 pagesTaylor Introms11GE PPT 03hddankerNo ratings yet
- My Watch Runs WildDocument3 pagesMy Watch Runs WildLarissa SnozovaNo ratings yet
- REMOVE CLASS 2024 SOW Peralihan MajuDocument4 pagesREMOVE CLASS 2024 SOW Peralihan MajuMohd FarezNo ratings yet
- What Is Robotic Process Automation?: ERP SystemDocument5 pagesWhat Is Robotic Process Automation?: ERP SystemAna BoboceaNo ratings yet
- MOW Procurement Management Plan - TemplateDocument7 pagesMOW Procurement Management Plan - TemplateDeepak RajanNo ratings yet
- Malabsorption and Elimination DisordersDocument120 pagesMalabsorption and Elimination DisordersBeBs jai SelasorNo ratings yet
- Fujitsu Lifebook p1120 ManualDocument91 pagesFujitsu Lifebook p1120 Manualمحمد يحىNo ratings yet
- Samsung RAM Product Guide Feb 11Document24 pagesSamsung RAM Product Guide Feb 11Javed KhanNo ratings yet
- Clock Al Ghadeer Setup GuideDocument4 pagesClock Al Ghadeer Setup Guideakberbinshowkat100% (2)
- Lecture01 PushkarDocument27 pagesLecture01 PushkarabcdNo ratings yet
- Tutorial Task 3 - A C P I WK 2Document8 pagesTutorial Task 3 - A C P I WK 2BM70621 Alya Zahirah Binti AziziNo ratings yet
- Atlas Ci30002Tier-PropanDocument3 pagesAtlas Ci30002Tier-PropanMarkus JeremiaNo ratings yet
- Ginglen 2022 - Necrotizing Enterocolitis - StatPearlsDocument8 pagesGinglen 2022 - Necrotizing Enterocolitis - StatPearlsBee GuyNo ratings yet
- Dedicated Teacher ResumeDocument2 pagesDedicated Teacher ResumeLei Pitallano ComboyNo ratings yet
- MFC-L2710DW 2Document8 pagesMFC-L2710DW 2Pinto ModakNo ratings yet
- M.Sc. Agriculture (Agronomy)Document23 pagesM.Sc. Agriculture (Agronomy)Abhishek MauryaNo ratings yet
- T WiZ60Document6 pagesT WiZ60leon liNo ratings yet
- Desk PiDocument21 pagesDesk PiThan LwinNo ratings yet
- Lodha GroupDocument2 pagesLodha Groupmanish_ggiNo ratings yet
- 01 A Brief Introduction To Cloud ComputingDocument25 pages01 A Brief Introduction To Cloud ComputingfirasibraheemNo ratings yet
- Ap4955 PDFDocument4 pagesAp4955 PDFGilvan HenriqueNo ratings yet
- Factory Test Report For OPzS 800 EED-20041724 2VDocument3 pagesFactory Test Report For OPzS 800 EED-20041724 2VmaherNo ratings yet
- 2iccas2005 Paper 377Document5 pages2iccas2005 Paper 377Cristian BandilaNo ratings yet